Columbia University
Irving Medical Center
Neurological Institute
710 West 168th Street, 3rd floor
(212) 305-1818
TaubCONNECT Research Perspectives:
September 2017
#2 An xQTL Map Integrates the Genetic Architecture of the Human Brain's Transcriptome and Epigenome
» #2 LTP and Memory Impairment Caused by Extracellular Aβ and Tau Oligomers is APP-Dependent
» #2 Neuropathologic Features of TOMM40 '523 Variant on Late-Life Cognitive Decline
» #2 An Approach to Studying the Neural Correlates of Reserve
» #1 Brain Atrophy Can Introduce Age-Related Differences in BOLD Response
» #2 Age-Related Biomarkers in LLFS Families With Exceptional Cognitive Abilities
» #2 Polygenic Risk Scores in Familial Alzheimer Diseases
» #1 Local Synthesis of Dynein Cofactors Matches Retrograde Transport to Acutely Changing Demands
» #3 Relation of Dysglycemia to Structural Brain Changes in a Multiethnic Elderly Cohort
» First Place: White Matter Changes in Alzheimer's Disease
» Alzheimer's Association International Conference (AAIC 2016)
» #1 Neuronal activity enhances tau propagation and tau pathology
» #2 Sleep Disordered Breathing and White Matter Hyperintensities in Community-Dwelling Elders
» #2 Dementia Risk and Protective Factors Differ in the Context of Memory Trajectory Groups
» #2 Parkinson's Disease: Guilt by Genetic Association
» #1 PDE5 Exists in Human Neurons and is a Viable Therapeutic Target for Neurologic Disease
» #1 Extracellular Tau Oligomers Produce An Immediate Impairment of LTP and Memory
» #3 Mediterranean Diet and Brain Structure in a Multiethnic Elderly Cohort
» #2 Novel Selective Calpain 1 Inhibitors as Potential Therapeutics in Alzheimer's Disease
» #1 First Place: DREADDs Activation in the Medial Entorhinal Cortex (MEC) of EC-Tau Mice
» #1 F-box/LRR-repeat protein 7 is genetically associated with Alzheimer's disease
» #2 The keystone of Alzheimer pathogenesis might be sought in Aβ physiology
» #1 Stereotaxic Infusion of Oligomeric Amyloid-beta into the Mouse Hippocampus
» #1 SUMO1 Affects Synaptic Function, Spine Density and Memory
» #2 Connectivity and Circuitry in a Dish Versus in a Brain
» #2 Mediterranean Diet and Leukocyte Telomere Length in a Multi-ethnic Elderly Population
» Neurotherapeutics: Rethinking Alzheimer's Disease Therapies
» #2 Olfactory deficits predict cognitive decline and Alzheimer dementia in an urban community
» Regulation of synaptic plasticity and cognition by SUMO in normal physiology and Alzheimer's disease
» Lobar Microbleeds Are Associated with a Decline in Executive Functioning in Older Adults
» Targeting Axonal Protein Synthesis in Neuroregeneration and Degeneration
» Coding mutations in SORL1 and Alzheimer's disease
» Axonally Synthesized ATF4 Transmits a Neurodegenerative Signal across Brain Regions
» Neurological disorders: Quality-control pathway unlocked
» Estrogen Receptor β Variants Modify Risk for Alzheimer's Disease in a Multiethnic Female Cohort
» Local synthesis of TC10 is required for membrane expansion during axon outgrowth
» Dynamin 1 is Required for Memory Formation
» Biobanked Alzheimer's Brain Tissue Yields Living Neurons
» Picomolar Amyloid-ß Peptides Enhance Spontaneous Astrocyte Calcium Transients
Stabilization of Dynamic Microtubules by mDia1 Drives Tau-dependent Aβ1-42 Synaptotoxicity
 |  |  | ||
| Francesca Bartolini, PhD | Xiaoyi Qu | Michael Shelanski, MD, PhD |
Alzheimer's disease is characterized by the presence in the brain of both amyloid plaques, containing the amyloid β peptide (Aβ), and neurofibrillary tangles, containing heavily phosphorylated forms of the microtubule-stabilizing protein tau. A common model posits that the accumulation of oligomeric Aβ plays a crucial synaptotoxic role in Alzheimer's disease, and hyperphosphorylated tau facilitates Aβ toxicity. The link between Aβ and tau, however, remains controversial.
Emerging evidence demonstrates that dynamic microtubules are critical regulators of neuronal homeostasis and synaptic function, suggesting that unwanted changes in microtubule dynamics and/or tubulin post-translational modifications, typically associated with microtubule longevity, may also be a trigger of neurodegenerative disease. Published online in the Journal of Cell Biology, a new study by the laboratory of Francesca Bartolini, including first author Xiaoyi Qu, with Dr. Michael Shelanski and colleagues from CUMC, found that synaptotoxic concentrations of Aβ acutely induce tubulin posttranslational modifications and stabilize dynamic microtubules in hippocampal neurons by reducing microtubule catastrophe frequency. Silencing or acute inhibition of the formin mDia1, a regulator of both actin and microtubule dynamics, suppresses these activities and corrects the synaptotoxicity and deficits of axonal transport induced by Aβ. The authors explored the mechanism of rescue and found that stabilization of dynamic microtubules promotes tau-dependent loss of synaptic density and tau hyperphosphorylation even in the absence of Aβ.
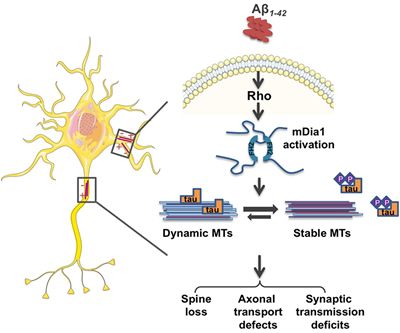
Figure 1: Working model for mDia1-mediated stabilization of dynamic MTs in Aβ-induced synaptotoxicity. Aβ induces the formation of "toxic" subsets of stabilized MTs through Rho signaling and mDia1 activation in neurons. Stabilized MTs cause a cellular stress response that leads to tau dissociation and hyperphosphorylation in the attempt to restore normal levels of dynamic and unmodified MTs. Induction of these neurotoxic pathways results in spine loss, axonal transport, and synaptic transmission deficits.
Collectively, these results indicate that Aβ may exert many of its neurotoxic effects by stabilizing dynamic microtubules through the activities of mDia1 and its activator RhoA, and demonstrate that inhibition of microtubule dynamics and accumulation of tubulin post-translational modifications are driving factors for the induction of tau-mediated neuronal damage (Figure 1). Based on this new model, the authors speculate that the Aβ-mediated loss of cognitive function in Alzheimer's disease could involve the progressive inhibition of microtubule dynamics at the early stages of memory formation when microtubules need to be highly dynamic to induce long-lasting changes in synaptic function. This would also contribute to the accumulation of hyperphosphorylated tau by triggering a cellular stress response aimed at restoring normal microtubule dynamics. Preventing changes in microtubule dynamics by inhibiting mDia1 could be a new therapeutic strategy to alleviate both Aβ and phospho-tau toxicity in Alzheimer's disease.
Francesca Bartolini, PhD
Assistant Professor of Pathology and Cell Biology
fb2131@cumc.columbia.edu
Xiaoyi Qu
Graduate Student/Pathobiology and Molecular Medicine
xq2126@cumc.columbia.edu
Michael Shelanski, MD, PhD
Henry Taub Professor of Alzheimer's Disease and the Aging Brain (in Pathology and Cell Biology and the Taub Institute)
mls7@cumc.columbia.edu
An xQTL Map Integrates the Genetic Architecture of the Human Brain's Transcriptome and Epigenome

Philip De Jager, MD, PhD, MMSc
Genome wide association studies (GWAS) have identified thousands of SNPs that are associated with various human diseases. However, most identified SNPs fall in the noncoding regions of the genome. Connecting these variants to regulatory changes in the expression of specific genes or to molecular pathways that may be implicated in human diseases is not straightforward. In addition, suggestive evidence indicates that many more such disease-related SNPs exist, but they are difficult to detect due to their typically small effect sizes and the challenge of multiple-testing burden in genome-wide assessment of common genetic variation. Expression quantitative trait locus (eQTL) analyses have been very useful in understanding the functional consequences of trait- and disease-associated variants and in identifying genes that are likely to be affected by a risk allele.
Recently, QTL analyses have been extended to other molecular phenotypes, such as DNA methylation (mQTL), and histone modification (haQTL). Overall, SNPs associated with molecular phenotypes (collectively, xQTLs) are over-represented among SNPs that are linked to various traits and diseases, and previous studies have used eQTL hits to prioritize associations in GWAS, leading to improved detection sensitivity. While a few data sets exist for brain tissue, large data sets measuring all three of these epigenomic and transcriptomic features have only recently been generated from the same brain region of each individual. Using one of the largest multi-omic data sets for brain tissue, Drs. Philip De Jager and Hans-Ulrich Klein, along with Drs. Sara Mostavi and Bernard Ng (University of British Columbia) and colleagues, generated a list of xQTLs as a Resource for the neuroscience community to further investigate the interplay between the genome, epigenome, and transcriptome in disease susceptibility.
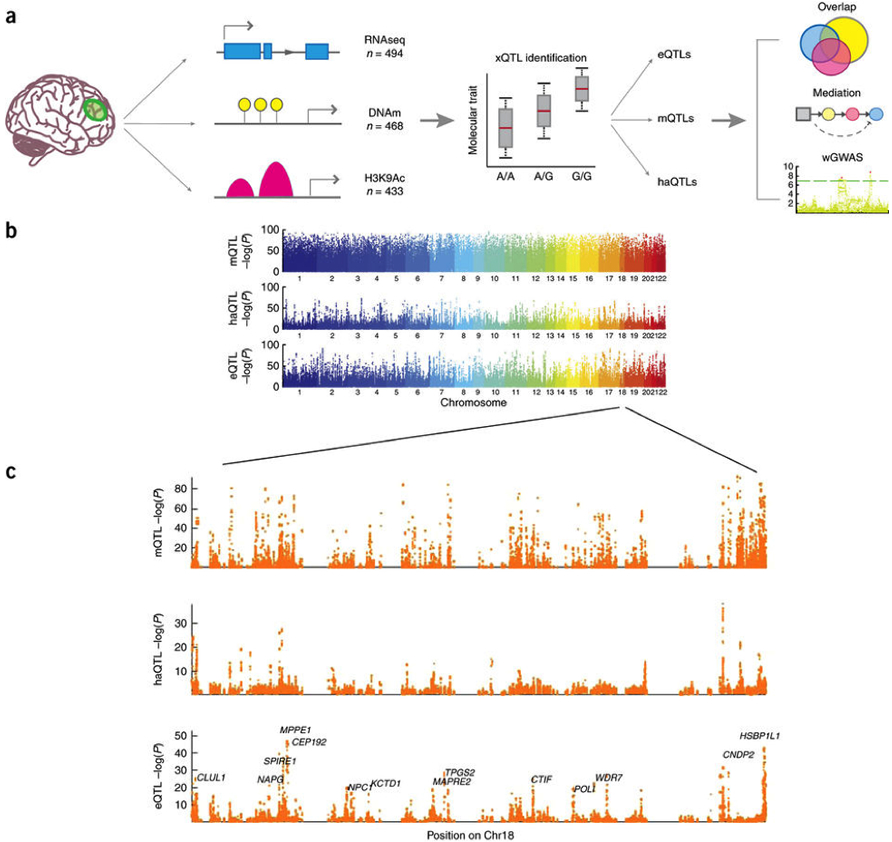 Figure 1: (a) Graphical summary of our data and analyses. We first associate genetic variation with each data type separately to establish our xQTL reference. We then use these xQTLs to assess whether a given SNP influences more than one data type, whether epigenomic features mediate the effects of SNPs on gene expression, and whether our xQTLs can be leveraged using a weighted GWAS (wGWAS) analysis approach to discover new susceptibility loci. (b) −log10 P-value of Spearman's correlation between SNPs and DNA methylation (mQTL), histone acetylation (haQTL) and gene expression (eQTL) vs. the SNPs' physical positions in the genome. Each dot represents the strongest association within a cis window for each SNP. (c) Zoomed-in Manhattan plot of chromosome 18 to illustrate P-value distribution of xQTLs at a higher resolution. |
Published online in Nature Neuroscience, they report that their list of xQTLs replicates well in both brain and blood data sets, but it also contains xQTLs that appear to be unique to the older brain. Notable biological insights drawn from this Resource include significant sharing of xQTL SNPs across the measured molecular phenotypes. Also, the effects of some eQTL SNPs on transcription are fully mediated by the epigenetic features, but further work and more data are needed to comprehensively assess the extent to which epigenomic features mediate eQTL effects in general. Further, Dr. De Jager and his colleagues use their xQTL reference to perform an xQTL-weighted GWAS and identify 18 new putative Schizophrenia susceptibility loci as well as two loci that may be involved in major depression. Thus, they have created a large new reference with which investigators can functionally annotate their results; enhance their analyses, as illustrated by their xQTL-weighted GWAS approach; and guide functional studies, as in their cell type analysis. This Resource can be easily accessed through the portal, xQTL Serve.
Philip De Jager, MD, PhD, MMSc
Professor of Neurology (in the Taub Institute, the Precision Medicine Initiative, and the Center for Translational and Computational Neuro-immunology)
pld2115@cumc.columbia.edu

