Columbia University
Irving Medical Center
Neurological Institute
710 West 168th Street, 3rd floor
(212) 305-1818
TaubCONNECT Research Perspectives:
March 2014

Ottavio Arancio, MD, PhD
Dynamin 1 is Required for Memory Formation
Dynamin activity is required for the maintenance of neurotransmitter-filled vesicle pool accounting for basal evoked neurotransmitter release in the central nervous system. However, a fundamental question is whether dynamin plays a role in higher functions such as in normal animal behavior. Now published in the journal, PLOS ONE, we have demonstrated that the activity of dynamin is permissive for cognitive function as it regulates and optimizes the synaptic neurotransmission efficiency in function of the neuronal activity level. Specifically dynamin 1 is key for both short- and long-term plasticity phenomena due to sustained activity while dynamin 2 and 3, the other two dynamin isoforms in the central nervous system do not compensate for the impairment of both memory and long-term potentiation due to selective dynamin 1 block by genetic ablation. The differential ability of the dynamin isoforms in modulating the neurotransmitter vesicle availability over time may be relevant in terms of release during fast stimulation and controlling plasticity underlying the onset of memory.
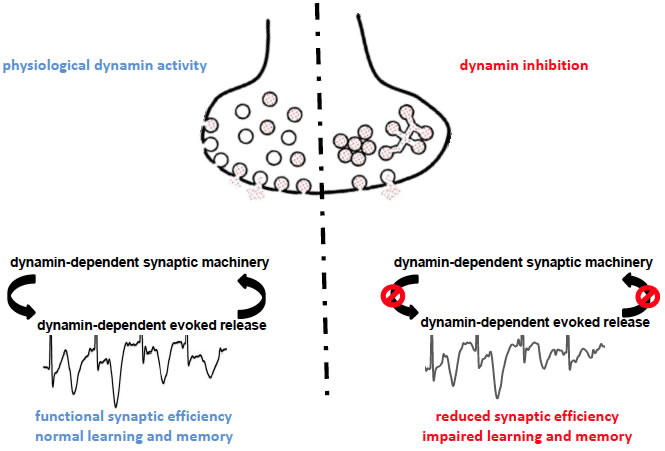
This schematic shows the two possible scenarios occurring at the release site in physiological conditions (left) and upon dynamin inhibition (right), according to present data. The membrane depolarization produced by sustained activity activates the exocytosis impinging on the dynamin-dependent evoked vesicle release pool. When dynamin can exert its functions (left), the dynamin-dependent evoked release pool undergoes in cycles of exocytosis/endocytosis ensuring maintain the release efficiency and the proper within-stimulation facilitation, necessary for plasticity. In conditions where dynamin content is reduced or its activity is inhibited (right), neurotransmitter vesicles tend to cluster in distal position with respect of the proper fusion site and the residual release-competent vesicles that still may fuse cannot ensure anyway the normal synaptic facilitation during rapid stimulation, leading to poor neuronal plasticity and impaired learning and memory.
Our findings are consistent with the non-obvious scenario in which the dynamin-dependent rapid use of synaptic vesicles sustains neurotransmission to ensure synaptic facilitation along repetitive stimulation. When dynamin is unable to contribute to the endocytotic recovery of vesicles or the clearance of synaptic release sites, the decreased vesicle availability or the impaired release machinery may affect the neuron capability for proper synaptic release. Given that memory formation is thought to be associated with changes in synaptic function occurring during repetitive stimulation, it is likely that dynamin 1 regulates memory formation through regulation of vesicle availability during sustained release.
Our discoveries are not only important because they highlight a novel basic mechanism regulating both synaptic plasticity and memory, but also because they open new perspectives for a function of dynamin 1 in pathology. Our findings lay the foundations for targeting dynamin in the cure of neurodegenerative diseases, such as Alzheimer's disease, where synaptic function is already impaired at the earliest phases of the disease. Interestingly, dynamin levels are decreased in the brains of Alzheimer's disease patients. While the possible link between the disease and dynamin has yet to be thoroughly characterized, one can hypothesize that reduced dynamin activity mediates, at least in part, the detrimental effects of beta-amyloid, a key protein in disease pathogenesis, on both synaptic plasticity and memory.
Ottavio Arancio, MD, PhD
Oa1@columbia.edu
Mauro Fà, PhD
mf2257@cumc.columbia.edu

