Columbia University
Irving Medical Center
Neurological Institute
710 West 168th Street, 3rd floor
(212) 305-1818
TaubCONNECT Research Perspectives:
April 2015
» #2 Mediterranean Diet and Leukocyte Telomere Length in a Multi-ethnic Elderly Population
» Neurotherapeutics: Rethinking Alzheimer's Disease Therapies
» #2 Olfactory deficits predict cognitive decline and Alzheimer dementia in an urban
community
» Regulation of synaptic plasticity and cognition by SUMO in normal physiology and
Alzheimer's disease
» Lobar Microbleeds Are Associated with a Decline in Executive Functioning in
Older Adults
» Targeting Axonal Protein Synthesis in Neuroregeneration and Degeneration
» Coding mutations in SORL1 and Alzheimer's disease
» Axonally Synthesized ATF4 Transmits a Neurodegenerative Signal across Brain Regions
» Neurological disorders: Quality-control pathway unlocked
» Estrogen Receptor ╬▒ Variants Modify Risk for Alzheimer's Disease in a Multiethnic
Female Cohort
» Local synthesis of TC10 is required for membrane expansion during axon outgrowth
» Biobanked Alzheimer's Brain Tissue Yields Living Neurons
» Biobanked Alzheimer's Brain Tissue Yields Living Neurons
» Picomolar Amyloid-╬▓ Peptides Enhance Spontaneous Astrocyte Calcium Transients
 |
 |
| Silvia Pasini, PhD | Michael Shelanski, MD, PhD |
Understanding the physiological mechanisms underlying learning and memory is of the utmost importance as the decline in these cognitive processes is a symptom of several neurodegenerative disorders, such as Alzheimer's disease, for which, at present, there are few effective treatments. The protein Activating Transcription Factor 4 (ATF4) has been of special interest in the context of memory formation, but its role has been controversial. Early studies suggested that this protein negatively regulates learning and memory. In contrast, more recent findings support the idea that ATF4 is a key player required for the formation of different types of memory. However, these studies were largely based on experiments in which alteration of ATF4 levels was achieved by either non-specific or indirect ways, opening the possibility that the observed findings might be due to the modulation of other proteins in addition to ATF4.
In a study published this month in Cell Reports, a team of investigators from the Taub Institute, including Silvia Pasini, Carlo Corona, and Jin Liu, led by Michael Shelanski and Lloyd Greene, specifically downregulated ATF4 levels in the adult mouse hippocampus, a brain area intimately involved in learning and memory formation and that deteriorates in Alzheimer's disease. They found that reducing ATF4 level leads to profound deficits in long-term memory, accompanied by significant impairments of two forms of synaptic plasticity, Long-Term Potentiation (LTP) and Long-Term Depression (LTD). LTP and LTD are widely considered to be cellular mechanisms of memory formation. In line with these findings, they also observed that lowering ATF4 levels affects excitatory neurotransmission between neurons, which is known to be crucial for synaptic plasticity.
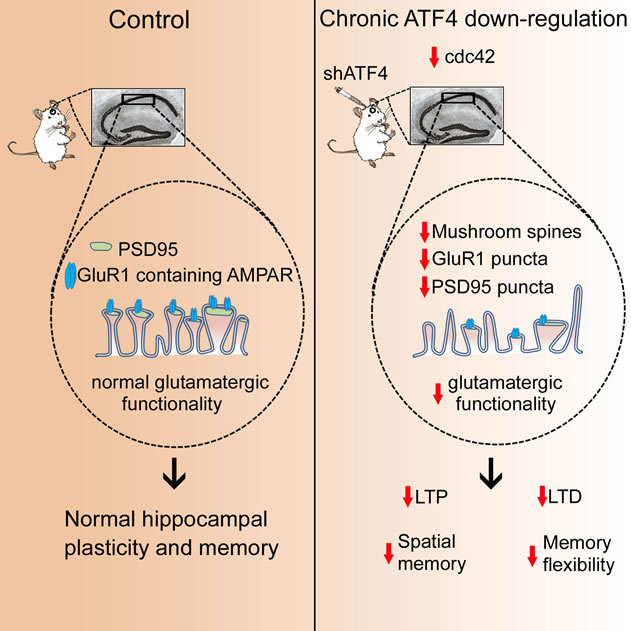 Schematic representation of what happens in the adult mouse hippocampus after ATF4 downregulation. Under basal conditions ATF4, by regulating levels of Cdc42, maintains shape and function of excitatory (glutamatergic) synapses, which ensures proper neuronal plasticity and memory. However, ATF4 reduction, by decreasing Cdc42 levels, induces a remodeling of synapse shape that, in turn, affects glutamatergic synaptic functionality eventually leading to deficits in synaptic plasticity and cognition.
|
This work is an extension of a previous study from the same team, in which they reported that ATF4 is a key modulator of post-synaptic development of synapses, through a mechanism that includes the alteration in the level of Cdc42, another important protein involved in the regulation of synapse shape and function.
Together, these findings suggest that ATF4 is a pivotal regulator of the physiological state necessary for learning and memory by maintaining the proper communication between neurons. Current efforts are underway to understand whether and how ATF4 plays an additional role in regulating inhibitory interactions between neurons. If this is the case, as anticipated, ATF4 will then represent a key modulator of the neuronal excitation/inhibition balance, deficits in which are thought to contribute to core symptoms of several neurodegenerative disorders. Such findings open the door to manipulations that change the levels of ATF4 in the brain so as to prevent or reverse the cognitive deficits associated with Alzheimer's and Parkinson's diseases.
Silvia Pasini, PhD
sp2938@cumc.columbia.edu
Michael Shelanski, MD, PhD
mls7@cumc.columbia.edu
Mediterranean Diet and Leukocyte Telomere Length in a Multi-ethnic Elderly Population
 |
 |  |
| Yian Gu, PhD | Lawrence S. Honig, MD, PhD | Nikolaos Scarmeas, MD, MS |
Telomeres are DNA sequences located at the ends of each chromosome. They provide an essential protective role for DNA. Because telomere sequences do not fully replicate during DNA replication, they become progressively shorter with each cell division. Thus, telomere length has been proposed to be a marker for biological aging. Shortened telomere length has been related with higher all-cause mortality, as well as a variety of age-related conditions such as cardiovascular disease, diabetes, and dementia.
Although telomere length is strongly influenced by genetic factors, telomere attrition might be modulated by environmental and lifestyle factors, including cigarette smoking, education, socioeconomic status, and diet. The Mediterranean-type diet (MeDi) is a healthy dietary pattern that has been associated with lower risk of a wide variety of diseases. The MeDi is characterized by high intake of vegetables, legumes, fruits, cereals, fish, monounsaturated fatty acids, low intake of saturated fatty acids, dairy products, meat and poultry, and mild to moderate ethanol use. The beneficial effects of MeDi might be related with its anti-oxidative and anti-inflammatory effects.
In a study published recently in the journal AGE, Drs. Yian Gu, Lawrence S. Honig, Nikolaos Scarmeas and collaborators from Taub, Sergievsky, and the departments of Neurology, Psychiatry, Medicine, and Epidemiology examined the relation between MeDi and telomere length among 1743 participants from the Washington Heights-Inwood Columbia Aging Project. MeDi was calculated from dietary information collected using a food frequency questionnaire. Telomere length was measured from leukocyte DNA. All the analyses were adjusted for age, sex, education, caloric intake, smoking, physical and leisure activities, in order to estimate the association of MeDi with telomere length that is independent of these factors.
In the overall study population, MeDi score was not associated with telomere length (╬▓=12.5; p=0.32), but higher intake (i.e. more than the middle level taken by the same gender from the study population) of vegetable and cereal consumption was associated with longer telomere length (╬▓=89.1, p=0.04) and shorter telomere length (╬▓=-93.5; p=0.03), respectively. Among non-Hispanic Whites, higher adherence to a MeDi was associated with longer telomere length (╬▓=48.3; p=0.05), and the results held after excluding dementia subjects (╬▓=49.6; p=0.05). Furthermore, lower intake of meat or dairy was associated with longer telomere length (╬▓=154.7, p=0.05; ╬▓=240.5, p<0.001, respectively) among non-Hispanic Whites.
Overall, the study found that higher adherence to a MeDi was associated with longer telomere length among Whites. Additionally, higher intake of vegetables but lower intake of cereal, meat, and dairy might be associated with longer telomere length among healthy elderly. The group has previously shown a beneficial role of MeDi in preventing Alzheimer's disease and cognitive decline. The results of the current study further support an important role of this diet in slowing down the aging process.
Yian Gu, PhD
Assistant Professor of Neurological Sciences (in Neurology, Epidemiology and the Taub Institute)
yg2121@columbia.edu
Lawrence Honig, MD, PhD
Professor of Clinical Neurology
lh456@columbia.edu
Nikolaos Scarmeas, MD, MS
Associate Professor of Clinical Neurology
ns257@columbia.edu

