Columbia University
Irving Medical Center
Neurological Institute
710 West 168th Street, 3rd floor
(212) 305-1818
TaubCONNECT Research Perspectives:
April 2014
and promotes axon regeneration through CNTF-mediated JAK/STAT signalling
» Local synthesis of TC10 is required for membrane expansion during axon outgrowth

Carol M. Troy, MD, PhD
Central nervous system (CNS) trauma can result in axonal injury. In the CNS axons fail to regenerate after injury, and such injuries often result in neuronal death. In order to restore functionality after injury, abrogation of neuronal death and axonal regeneration are both necessary. Many factors have been implicated in blocking regeneration but effective regeneration has not previously been obtained.
Now published in the journal BRAIN, we show that suppressing multiple caspases—enzymes that mediate cell death—protects retinal ganglion cells from apoptosis and promotes axonal regeneration. In a model of optic nerve crush, combined suppression of caspase-2 and caspase-6 rescues more than 95% of the retinal ganglion neurons from death and promotes significant axon regeneration. Regeneration is approximately 10 fold greater than what was found in prior work. Our studies suggest that our intervention promotes axonal regeneration via induction of glial activation and the release of the growth factor CNTF, which promotes axon regeneration in neurons. We do not yet know the proteolytic target of caspase-6 that mediates the regulation of glia.
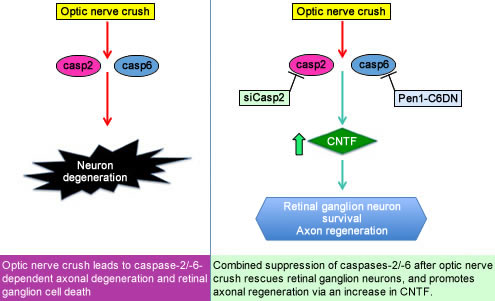
Caspases, a multi-membered family of proteases, are mainly known for their role in apoptosis. Resolving the function of individual caspases has been hindered by the wide-spread use of non-specific compounds and methods. In our studies, we have used genetic and molecular approaches to target individual caspases. We have also designed protein inhibitors that penetrate cells and modulate the activity of specific caspases. These can be delivered to the CNS intravitreally and intranasally. With these novel tools, we are beginning to understand that certain caspases have non-apoptotic functions in the adult nervous system.
Our studies are important as they establish a potential therapeutic approach for the treatment of CNS trauma. They also illustrate a novel non-apoptotic function of caspase-6 in the regulation of glial activation and release of cytokines and CNTF. Glial activation is found in many neurologic diseases, and this new function of caspase-6 may provide insights into the mechanisms of these diseases.
Carol M. Troy, MD, PhD
cmt2@cumc.columbia.edu
Nsikan Akpan, PhD
nsikanakpan@gmail.com
Local Synthesis of TC10 is Required for Membrane Expansion During Axon Outgrowth

Ulrich Hengst, PhD
During the development of the nervous system, axons are guided to their targets by extracellular cues that direct the motion of the growth cones at the tip of axons. Within these highly motile structures signaling pathways triggered by repulsive or attractive guidance cues are integrated to establish the orientation and growth rate of the axons. Two basic mechanisms in distal axons and growth cones are required for this process: rearrangement of the cytoskeleton and the addition or removal of membrane at specific sites within the growth cone. These two events have to occur synchronously, at the same sites within growth cones. Currently, however, it is only partially understood how cytoskeletal and membrane dynamics are integrated. We proposed that the local, 'on-demand' synthesis of proteins that are pivotal in their respective pathways might be a unique way to ensure temporal and spatial coincidence of seemingly parallel pathways.
The phospholipids for the new membrane are produced in the Golgi apparatus in the cell body from where they are transported as empty membrane bound vesicles called plasmalemmal precursor vesicles (PPVs). The sites of exocytosis of PPVs are marked by protein complexes at the membrane, one of which is the exocyst. We wondered how the activity of the exocyst would be directed to specific sites on the membrane. We focused on the small cdc42-like GTPase TC10 which is required for exocytosis, and hypothesized that stimulus-dependent synthesis of TC10 at precise locations in the growth cone would tag these sites for the fusion of PPVs with the plasma membrane and membrane expansion.
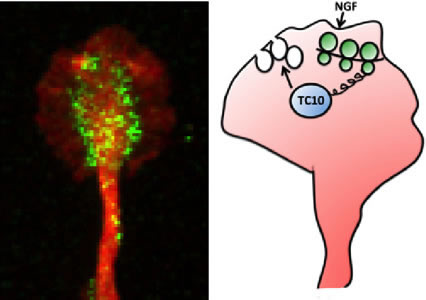
Figure: (left) Immunofluorescence staining for TC10, green, and tubulin, red, in the growth cone of an embryonic rat dorsal root ganglion neuron. (right) Scheme depicting the discovered signaling mechanism. NGF triggers the locally restricted translation of TC10 mRNA within growth cones, leading to a spatially confined increase of TC10 protein abundance facilitating the fusion of PPVs with the growth cone membrane.
In our study, published recently in Nature Communications, we demonstrate that a guidance cue like nerve growth factor (NGF) induces axonal synthesis of TC10. This rapid increase (occurring within 10 minutes) in axonal TC10 can be inhibited by transfection of axons with siRNA targeted to TC10 mRNA proving that axonal synthesis of this protein relies on the localization of TC10 mRNA in axons. Furthermore, we show that inhibition of local synthesis of TC10 blocks NGF-induced exocytosis of PPVs in the growth cone and NGF-induced axon growth. Notably, the levels of TC10 mRNA in axons are just slightly above the currently available detection methods, yet we show that even these minuscule amounts of mRNA are essential for important functions like axon outgrowth. Lastly, we observed that axonal synthesis of TC10 is co-regulated with that of Par3 (a key player in the cytoskeletal rearrangement process) downstream of PI-3K, Rheb or mTOR.
Our study highlights two important concepts. Firstly, we demonstrate that the low abundance of a specific mRNA within axons does not necessarily diminish its biological significance and that axonal protein synthesis is not meant to provide bulk protein but rather fulfills a spatially restricted and temporally acute demand for greater protein levels within growth cones. Secondly, we provide an example of proteins (TC10 and Par3) whose co-regulated local synthesis triggers the coordinated action of two parallel pathways to achieve the common goal of axon growth. Local protein synthesis thus provides axons with a degree of semi-autonomy from their cell bodies enabling rapid adjustments to changed extracellular conditions. Ongoing research in our group addresses the question of how disruptions of this process contribute to neurodegeneration.
Ulrich Hengst, PhD
uh2112@cumc.columbia.edu
Neilia G. Gracias, PhD
ng2373@columbia.edu

