Columbia University
Irving Medical Center
Neurological Institute
710 West 168th Street, 3rd floor
(212) 305-1818
TaubCONNECT Research Perspectives:
November 2014
» Inbreeding among Caribbean Hispanics from the Dominican Republic and its effects on risk
of Alzheimer disease
» Coding mutations in SORL1 and Alzheimer's disease
Targeting Axonal Protein Synthesis in Neuroregeneration and Degeneration
 |  | |
| Ulrich Hengst, PhD | Jimena Baleriola Gomez De Pablos, PhD |
Neurons are the most highly morphologically polarized cells, with axons and dendrites that extend over macroscopic distances far exceeding the diameter of the their cell bodies. This unique property seems to require adaptions in the way nerve cells organize their metabolism and signaling events. At the end of the 19th century, it was proposed that neurons might synthesize at least part of their building blocks, such as proteins, locally, within the axons and dendrites. This idea is now widely accepted in the case of dendrites, where local protein synthesis is required for the specific remodeling of individual synapses and, thus, for memory and learning. In contrast, the fact that neither the protein synthesis machinery nor the templates, i.e. ribosomes and mRNAs, are readily detectable in axons in the mature brain has led to the widely held believe that axons are incapable of producing protein locally.
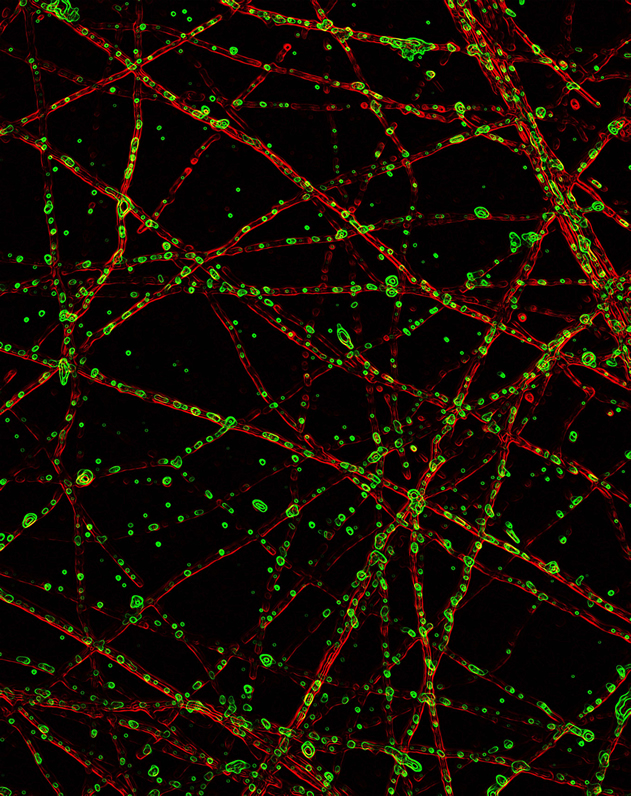
Immunostaining reveals the presence of ATF4 (green) in β-amyloid treated axons (red) of hippocampal neurons. Axonal ATF4 expression is required for the retrograde spread of Aβ-dependent neurodegeneration and is a potential target to interfere with disease progression. (Image credit: Jimena Baleriola, PhD)
Over the last 15 years, work by several groups, including our own, has begun to change this notion and to establish the important role of intra-axonal protein synthesis during the development of the nervous system. Mature axons indeed have a greatly reduced capacity for protein synthesis but, importantly, upon nerve injury or in response to neurodegenerative stimuli, the situation changes drastically: within a very short time, ribosomes and mRNAs are being recruited into axons and a subset of proteins is synthesized locally. While the synthesis of some of these proteins ameliorates the situation and supports nerve regeneration after nerve injury, other proteins appear to be detrimental and facilitate degeneration or the failure of injured axons to regrow. The realization that axonal protein production can have both positive and negative effects in a pathological situation opens up the possibility that interference with this process might be a novel and underappreciated way to manipulate neuron intrinsic pathways of axonal regeneration and neurodegeneration.
In our recently published Neurotherapeutics review article, we summarize the current state of knowledge, evaluate the available molecular tool set for both preventing and boosting local production of specific proteins, and discuss remaining challenges and problems that should be addressed to be able to judge the translational potential of targeting intra-axonal protein synthesis.
Ulrich Hengst, PhD
Uh2112@cumc.columbia.edu
Jimena Baleriola Gomez De Pablos, PhD
Jb3275@cumc.columbia.edu
 |
 |
| Badri Vardarajan, PhD | Joe Lee, DrPH |
A critical barrier to lessening the impact of Late Onset Alzheimer’s disease (LOAD) is the slowed development of drugs that prevent or treat LOAD due, in part, to an incomplete understanding of the biology of the disease. Determining genes and networks that contribute to Alzheimer’s disease (AD) could reveal insights about the etiology of the disease for development of drugs and diagnostic techniques to identify subjects at risk. Gene discovery is particularly powerful in densely phenotyped consanguineous families multiply affected by AD. Our group, the Laboratory for Genetic Epidemiology, has identified Caribbean Hispanic families that have been have been characterized, sampled and followed over the past two decades as a part of the Estudio Familiar de Influencia Genetica en Alzheimer (EFIGA) study. In an earlier study published in JAMA Neurology, we determined the incidence of new AD cases in family members of probands was 5 times the incidence in outbred Caucasian populations. Now online in Genetics in Medicine, we tried to estimate the levels of inbreeding within this population and its effects on AD risk. Using genome-wide SNP data, we calculated the inbreeding by adjusting for admixture. We assumed that Caribbean Hispanic populations were derived from 3 to 5 ancestral populations and, by adjusting for the excess homozygosity conferred by admixture, we estimated the true inbreeding levels in the population. We observed that the inbreeding in Caribbean Hispanic population ranges from F=0.002 (±0.018) to 0.018 (±0.048) with and without adjusting for admixture. This implies that on an average the two unrelated subjects share genetic material similar to third cousins. We also observed that degree of inbreeding is a significant predictor of AD risk (p=0.03). This degree of inbreeding largely increases the a priori power to identify causative variants.
Coding mutations in SORL1 and Alzheimer's disease
Building on this powerful study design, we developed a deep sequencing study to find rare mutations in the SORL1 gene. SORL1 was previously identified by our group as being both genetically and functionally associated with LOAD. In our earlier studies and subsequent replication studies, common polymorphisms (mostly intronic) have been associated with LOAD. However, functional variants, which are more likely to play a functional role in disease biology, have not been identified in SORL1. Recently published online in the Annals of Neurology, we conducted targeted deep sequencing of the SORL1 gene in Caribbean Hispanic and Caucasian subjects from Toronto. We identified three variants in SORL1 that showed nearly complete segregation within families and were deemed deleterious by bioinformatics analysis in Caribbean Hispanic families multiply affected by LOAD. Variants rs117260922 and rs143571823 were rare (maf<1%), but rs2298813 was more frequent (maf=15.6%). In cell culture studies using transfected HEK cells, these mutations produced more Aβ 40 and Aβ 42 compared to the wild type and all three bound to APP less efficiently than the wild type protein. All mutants increased the amount of the APP at the cell surface, which failed to direct full-length APP into the retromer-recycling endosome pathway. Targeted re-sequencing of SORL1 in 211 LOAD cases of Northern European ancestry identified 13 rare missense variations and a deletion of conserved residue p.N174 of which seven overlapped with those detected in the Caribbean Hispanic cases, including rs117260922 and rs2298813.
Richard Mayeux, MD, MSc (PI)
Laboratory for Genetic Epidemiology
Badri Vardarajan, PhD
Bnv2103@cumc.columbia.edu
Joe Lee, DrPH
Jhl2@cumc.columbia.edu

