Columbia University
Irving Medical Center
Neurological Institute
710 West 168th Street, 3rd floor
(212) 305-1818
TaubCONNECT Research Perspectives:
November 2015
» #2 Novel Selective Calpain 1 Inhibitors as Potential Therapeutics in Alzheimer's Disease
 |  | |
| Christian Habeck, PhD | Yaakov Stern, PhD |
As recently published in NeuroImage, a new study by Drs. Christian Habeck, Yaakov Stern, and colleagues analyzed the data of 191 normal adults in the age range 20 to 77 who were imaged in a functional Magnetic Resonance Imaging (fMRI) scanner while performing 12 cognitive tasks. The tasks were chosen to represent the 4 cognitive "Reference Abilities" (estimated with 3 different tasks per ability): (1) memory, (2) fluid reasoning, (3) perceptual speed, and (4) vocabulary. The first three of the abilities have generally been observed to decline with aging, while the fourth usually stays unchanged or even improves with aging. For the fMRI data, after some pre-processing and linear time-series modeling, the investigators had one activation map per participant for each of the 12 tasks available. They applied a relatively simple mathematical technique to the activation maps called "linear indicator regression" to derive four activation patterns underlying the four Reference Abilities. The derivation was only performed in the activation maps of the participants aged 30 and younger. Habeck et al. reasoned that the restriction to young adults would allow them to achieve the best possible manifestation of the underlying Reference Ability Neural Networks (RANNs), without any influence of aging.
The activation patterns, although derived in the young participants, can be applied prospectively to the activation maps of the middle-aged and older participants (aged 31 and above.) A simple test of the validity of these patterns is whether they can accurately classify the activation maps into the correct Reference Ability. This means that for each activation map, one of four labels (1=memory, 2=fluid reasoning, 3=perceptual speed, and 4=vocabulary) can be predicted, depending on the observed topographic similarities of the activation map in question, with each of the four activation patterns derived in the young participants. Somewhat surprisingly, Habeck et al. found a high classification accuracy of 81% in the middle-aged and older participants, much better than the chance level of 25% (1 out of 4 abilities). Classification accuracy could also be computed on an individual-participant level and was found not to be associated with individual participant's age in the people aged 31 and older. However, classification accuracy was associated with verbal intelligence and total brain volume, such that participants with higher verbal intelligence and higher brain volume also showed higher classification accuracy, which implies better specificity of the derived networks to their associated cognitive processes. For each of the four activation patterns and each participant, the overall level pattern utilization can also be computed which likewise did not show any association with individual participant's age.
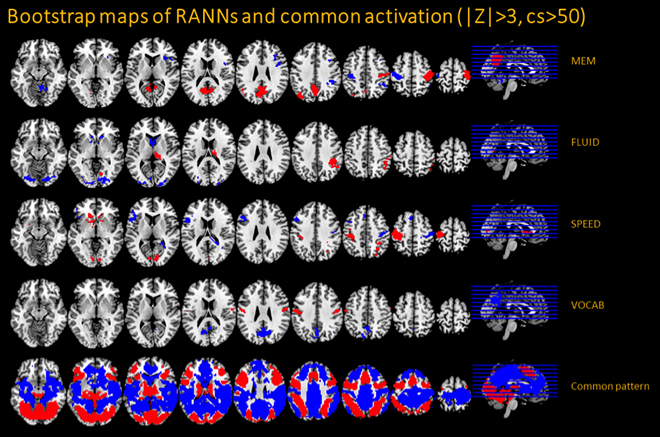 Figure above: 4 activation patterns capturing the Reference Abilities: (1) memory (=MEM), (2) fluid reasoning (=FLUID), (3) perceptual speed (=SPEED), and (4) vocabulary (VOCAB). The lowest row shows an activation pattern common to all tasks. Red color indicates increased activation relative to a rest condition where participants viewed a blank screen; blue color indicates decreased activation.
|
These findings imply that the networks underlying basic cognitive processes (at least as derived in the current study framework), and their manner of operating, are stable across the adult lifespan. The authors suggest that, with additional data, these networks can be derived for each age decade, which allows the observation of topographic changes with age and the influence of structural brain variables, like cortical volume, cortical thickness and amyloid deposition. From a translational standpoint, the networks might also hold diagnostic and prognostic power above and beyond brain-structural health for predicting cognitive dysfunction.
Christian Habeck, PhD
Associate Professor of Neuroimaging (in Neurology and in the Taub Institute)
ch629@cumc.columbia.edu
Yaakov Stern, PhD
Professor of Neuropsychology (in Neurology, Psychiatry, the Sergievsky Center, and the Taub Institute)
ys11@cumc.columbia.edu
Novel Selective Calpain 1 Inhibitors as Potential Therapeutics in Alzheimer's Disease

Ottavio Arancio, MD, PhD
The vast majority of failing clinical efforts against Alzheimer's disease is directed to the problem of amyloidogenic protein deposition, because proteinaceous aggregates consisting of deposition of extracellular amyloid plaques and intracellular neurofibrillary tangles are the major histopathological hallmarks of the disease. Recently published in the Journal of Alzheimer's Disease, a new study by the laboratory of Dr. Ottavio Arancio, including colleagues from the Taub Institute and other academic institutions, focuses, in turn, on preserving synaptic functionality. Such an approach is justified by ample evidence suggesting that Alzheimer's disease starts as a synaptic disorder, and is potentially effective in all diseases involving hyper-activation of the calpain system. Previous work by the Arancio Laboratory found that inhibition of calcium-dependent enzymes called calpains improved memory and synaptic transmission in a mouse model of Alzheimer's disease (AD). This led to an effort to translate findings on the etiopathogensis of AD, and on possible useful strategies to counteract the disease, into therapeutics.
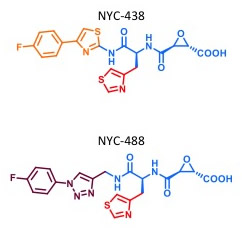
NYC438 and NYC488 chemical structures.
In the current study, Fà et al. were able to validate the high therapeutic potential of two novel chemical entities inhibiting calpains, known as NYC438 and NYC488. They used a strategy including a phenotypical screening of calpain inhibitors developed via medicinal chemistry refined through a target-based computational approach. The inhibitors were screened using a synaptic assay that demonstrated that the novel compounds are capable of restoring normal communication among cells in the brain. Most importantly, the novel molecules were effective in restoring learning and memory in a mouse model of Alzheimer's disease, while they were devoid of toxic effects even when administered chronically at doses largely higher than the minimal effective dose (at least > 10-fold, according to the FDA recommendations). The availability of a new series of effective, non-toxic therapeutics, exerting their activity on the molecular mechanisms of the disease, is a positive step toward a better cure for Alzheimer's disease and any other disease associated with calpain overactivation.
Ottavio Arancio, MD, PhD
Associate Professor of Pathology and Cell Biology (in the Taub Institute)
Oa1@cumc.columbia.edu