Columbia University
Irving Medical Center
Neurological Institute
710 West 168th Street, 3rd floor
(212) 305-1818
TaubCONNECT Research Perspectives:
May 2017
#2 An Approach to Studying the Neural Correlates of Reserve

Ottavio Arancio, MD, PhD
"All my work would be incomplete without the goal to move each project forward to the stage where it not only provides new biological insights but also, when appropriate, serves as the basis for future development of new therapeutic strategies," says Taub investigator Dr. Ottavio Arancio. To this end, Dr. Arancio has enhanced his translational research efforts through collaborations with medicinal-chemists, biotechnology specialists, pathologists, and clinicians. The ultimate goal of the studies in the Arancio laboratory has been to design novel therapeutic approaches that might be effective in preventing or delaying disease onset.
Increase in the levels of Aβ peptide in the brain is viewed as a relevant early mechanism in Alzheimer’s disease (AD) etiopathogenesis. However, Aβ lowering therapies have so far failed to enter the market. One possible explanation for these failures is in the discovery from Dr. Arancio’s lab that Aβ does not only disrupt memory when it is in high amounts in the brain, but is also necessary for formation of memory in the brain of normal healthy individuals. Thus, acting at the downstream level of Aβ elevation might be used as an alternative strategy for treating AD. Based on these findings, several laboratories, including Dr. Arancio's group, have investigated memory-relevant molecular pathways that are down-regulated following Aβ elevation. One such pathway is the cyclic adenosine monophosphate (cAMP) cascade, including a series of enzymes that destroy cAMP, called phosphodiesterases (PDEs). Among the 11 different PDEs, the type 4 (PDE4) family, comprising four isoforms (PDE4A-D), has been identified as one of the most promising targets for the treatment of cognitive-related disorders, with the hypothesis that PDE4 inhibition might be beneficial in AD.
Pan-PDE4 inhibitors, such as rolipram, are effective, pro-cognitive drugs in pre-clinical settings but have severe, undesired side effects (i.e. nausea and vomit). Given this, the Arancio laboratory teamed-up with the labs of Drs. Ernesto Fedele and Olga Bruno at the University of Genoa in Italy and Dr. Jos Prickaerts at Maastricht University in the Netherlands to test the biological efficacy of GEBR-32a, a new, specific PDE4 inhibitor acting against the "D" isoform that should not be involved in the emetic effects by PDE4 inhibition. As published in Scientific Reports, they found that the compound efficiently enhances cAMP in neuronal cultures and hippocampal slices, and is rapidly distributed within the central nervous system. Most importantly, the compound ameliorated memory and its synaptic surrogate long-term potentiation in a mouse model of Aβ elevation. Of great relevance, their toxicological analysis indicated that GEBR-32a is not cytotoxic and genotoxic, and did not seem to possess emetic-like side effects.
The present findings open up the possibility of exploiting the use of PDE4 inhibitors in clinical trials against memory loss in Alzheimer’s disease.
Ottavio Arancio, MD, PhD
Professor of Pathology and Cell Biology (in the Taub Institute)
oa1@cumc.columbia.edu
An Approach to Studying the Neural Correlates of Reserve

Yaakov Stern, PhD
Dr. Yaakov Stern and his Cognitive Neuroscience of Aging Laboratory have been systematically examining and developing models of cognitive reserve since Dr. Stern first introduced the concept back in the early 1990s. A seminal work by Dr. Stern titled, "Cognitive Reserve in Ageing and Alzheimer's disease," was published in The Lancet Neurology in 2012, and named #1 of the "Top 50 Most Influential papers" in AD research by the Journal of Alzheimer's Disease in 2016. Published more recently in Brain Imaging and Behavior, Dr. Stern reviews his current understanding of the concepts of cognitive reserve (CR), brain reserve (BR), and brain maintenance (BM), and describes his group’s approach to using imaging to study the neural basis of these concepts.
Their working hypothesis, according to Dr. Stern, is that CR operates through individual differences in how tasks are processed in the brain. In addition, their work incorporates the concept of BR, in order to account for individual differences in measures of integrity of the brain itself. The concept of BR has taken on greater importance, Dr. Stern explains, because of increasing evidence that life exposures can reshape the brain and maintain brain integrity, a process called BM.
Dr. Stern presents a working model for utilizing data regarding brain integrity, clinical status, cognitive activation, and CR proxies to develop analyses that can explore the neural basis of cognitive reserve and brain maintenance (see figure 1).
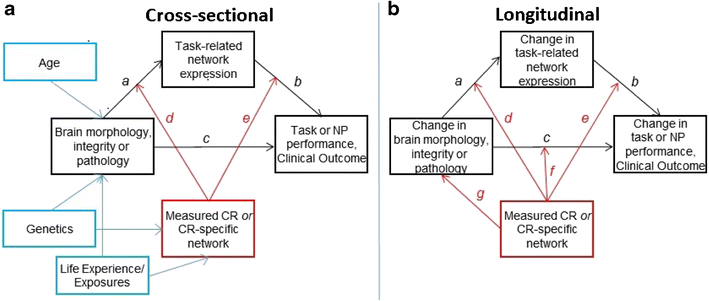 Figure 1: Cross-sectional (a) and longitudinal (b) models for studying cognitive reserve (CR), brain reserve (BR) and brain maintenance (BM). The models are discussed in-depth in the text. Letters are for identification only and do not have any mathematical significance. |
In Fig. 1a, the current status of the brain is characterized by measures of brain morphology and integrity, as well as AD pathology. Their cross-sectional model incorporates BR but not BM, because BM cannot be directly observed in cross-sectional data. "In our studies," explains Dr. Stern, "we try to incorporate as many imaging modalities as possible to characterize the status of the brain. These include measures of brain volume and cortical thickness, white matter integrity, resting cerebral blood flow and white matter hyperintensity burden. We are acquiring measures of resting BOLD networks, such as those associated with the default mode network. It is an open question whether these networks belong in our characterization of the status of the brain, or should be more accurately considered neural along with fMRI data as components of the neural implementation of CR."
Figure 1b addresses how prospectively collected data can allow the investigators to incorporate change over time in all of their measures and better address models of CR, BR and BM.
According to Dr. Stern, the basic model assumes that the effect of brain changes on cognition is mediated by task-related activation. "We treat CR as a moderator to understand how task-related activation might vary as a function of CR, or how CR might operate independently of these differences in task-related activation. My hope is that this presentation will spark discussion across groups that study these concepts, allowing us to come to some common agreement on definitions, methodology and approaches," says Dr. Stern.
Yaakov Stern, PhD
Professor of Neuropsychology (in Neurology, in Psychiatry, in the Gertrude H. Sergievsky Center and in the Taub Institute for Research on Alzheimer's Disease and the Aging Brain) at CUMC
Director, Division of Cognitive Neuroscience
ys11@cumc.columbia.edu

