Columbia University
Irving Medical Center
Neurological Institute
710 West 168th Street, 3rd floor
(212) 305-1818
TaubCONNECT Research Perspectives:
January 2014
» Picomolar Amyloid-β Peptides Enhance Spontaneous Astrocyte Calcium Transients
Biobanked Alzheimer's Brain Tissue Yields Living Neurons
Faithfully recapitulating Alzheimer's disease using model systems in the laboratory is a key roadblock to making progress in combatting this disease. Genetically modified animals have proven enormously useful, but they can only take you so far because Alzheimer's disease stems from the interactions of many genes.
Researchers are exploring a new approach using cultured skin cells from living human patients that are reprogrammed into stem cells. These induced pluripotent stem cells (iPSCs), which have the full human genome, are promising because they can be prodded into becoming neurons that can then be used for mechanistic studies and drug screening. But there is a problem. Diagnosing Alzheimer's disease requires an autopsy, the gold standard for diagnosis and grading disease severity, and researchers working on iPSCs need to know the diagnosis of the patient from which their cells are derived.
It occurred to Taub faculty member John Crary, MD, PhD, that it might be possible circumvent this problem using the extensive collection of tissues in the Taub Institute brain bank. The challenge was that the brain tissue in the bank had not been harvested with stem cell generation in mind – importantly it had not been preserved with cryoprotectants which are essential for preserving cells during freezing. Further, much of the tissue has been stored in the freezer for many years, and it was unclear whether it would be possible to recover living cells to serve as the source for making iPSCs.
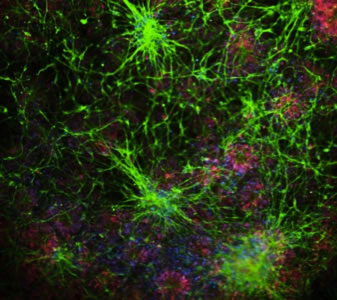
Microscopic image of neurons growing in culture (green) made from the dura mater of an autopsy-confirmed Alzheimer's disease patient.
Dr. Crary teamed up with Scott Noggle, PhD, co-senior investigator and the Charles Evans Senior Research Fellow for Alzheimer's Disease at the New York Stem Cell Foundation (NYSCF). They chose to target the dura mater, the tough outer covering of the brain, a region they suspected may harbor cells that might survive the freezing process. With a lot of patience, they were able to grow dural fibroblasts and reprogram them into iPSCs and ultimately convert them into living neurons. Remarkably, they were even successful in growing neurons from archival tissues that had been frozen for over 11 years. They also created iPSCs from patients with Huntington disease, Parkinson disease, amyotrophic lateral sclerosis and multiple system atrophy. These results were published this January in the journal Acta Neuropathologica Communications. NYSCF researchers Andrew Sproul, PhD and Lauren Vensand are the co-first authors of the study.
This advance is important because it will allow Taub Institute scientists to leverage the brain bank in a powerful new way, dramatically accelerating their ability to create iPSC models for Alzheimer's disease and the related neurodegenerative disorders.
John Crary, MD, PhD
jc2892@columbia.edu
Picomolar Amyloid-β Peptides Enhance Spontaneous Astrocyte Calcium Transients
Amyloid-β (Aβ) peptides are notorious in Alzheimer's disease (AD) research as the molecular culprits central to disease pathogenesis. However, recent research* from Ottavio Arancio of the Taub Institute, and others, indicates that the peptide may also have a Dr. Jekyll side: at physiologically-relevant low picomolar concentrations, Aβ can have positive modulatory effects on synaptic plasticity and memory. A new study by Lee et al. from the Arancio lab, published in the Journal of Alzheimer's Disease, follows up on these findings and points to astrocytes as potential cellular mediators of picomolar Aβ signaling. Using primary astrocyte cultures, the team discovered that acute application of 200 pM Aβ enhances spontaneous intracellular calcium transients. This type of calcium signaling represents a primary mechanism of astrocyte activation and has been observed in vivo, with functional impact on neural physiology and neurotransmission. The Aβ-induced enhancement was observed as long-term increases in both the frequencies and amplitudes of the astrocyte calcium transients. The enhancement effects were dependent on α7 nicotinic acetylcholine receptors, which have been demonstrated to bind Aβ with high picomolar affinity. Pharmacological inhibition or genetic deficiency of the receptor blocked the picomolar Aβ-induced potentiation of astrocyte calcium signaling. Given the accumulating evidence for astrocyte involvement in neurotransmission, the current findings indicate that Aβ may have a physiological function in regulating neuron-glia signaling. Furthermore, dysfunction of this signaling process may contribute to astrocyte-related components of AD pathology.
Ottavio Arancio, MD, PhD
Oa1@columbia.edu
Linda Lee, PhD
LindaLee@caa.columbia.edu
*Picomolar amyloid-beta positively modulates synaptic plasticity and memory in hippocampus.
Puzzo D, Privitera L, Leznik E, FÃ M, Staniszewski A, Palmeri A, Arancio O.
J Neurosci. 2008 Dec 31;28(53):14537-45. doi: 10.1523/JNEUROSCI.2692-08.2008.
PMID: 19118188 [PubMed - indexed for MEDLINE]
*Endogenous amyloid-β is necessary for hippocampal synaptic plasticity and memory.
Puzzo D, Privitera L, Fa' M, Staniszewski A, Hashimoto G, Aziz F, Sakurai M, Ribe EM, Troy CM, Mercken M, Jung SS, Palmeri A, Arancio O.
Ann Neurol. 2011 May;69(5):819-30. doi: 10.1002/ana.22313. Epub 2011 Apr 6.
PMID: 21472769 [PubMed - indexed for MEDLINE]

