Columbia University
Irving Medical Center
Neurological Institute
710 West 168th Street, 3rd floor
(212) 305-1818
TaubCONNECT Research Perspectives:
February 2016
» #2 Older Adults with Poor Self-rated Memory have less Depressive Symptoms and Better Memory Performance when Perceived Self-efficacy is High
 |  | |
| Natura Myeku, PhD | Karen Duff, PhD |
There is a significant and urgent need to find novel therapies that combat the accumulation of proteins that cause neurodegenerative diseases. The ubiquitin proteasome system (UPS) is the major pathway that degrades abnormal, misfolded proteins, including those implicated in diseases such as Alzheimer's disease (AD), Parkinson's disease (PD) and FrontoTemporal Degeneration (FTD). In a normally functioning cell, proteins are covalently tagged by the attachment of a polyubiquitin chain leading to rapid binding and hydrolysis by the 26S proteasome. The accumulation of ubiquitinated protein inclusions in neurodegenerative diseases suggests that defects exist in 26S proteasome-mediated clearance. In the Taub Institute laboratory led by Dr. Karen Duff, one of the proteins that we have studied, aggregated tau, is a feature of several neurodegenerative diseases, including AD and FTD. Tau aggregates are polyubiquitinated and several studies have implicated UPS dysfunction in response to aggregated tau accumulation, which gives rise to a pathology known as tauopathy. Currently, there are no drugs that stop, delay, or slow down disease progression associated with tauopathy.
In the present study by Myeku et al., published in Nature Medicine, we elucidated how proteasome function is progressively impaired with worsening tauopathy due to the physical association of proteasome components with tau aggregates and oligomers. These aggregates are thought to obstruct or "choke" the entrance to the proteasome, preventing subsequent protein degradation. Proteasomes from a mouse model of tauopathy failed to degrade ubiquitinated proteins in vivo and in vitro, strongly suggesting that dysfunctional proteasome can exacerbate aggregate accumulation, proteotoxicity, and disease progression.
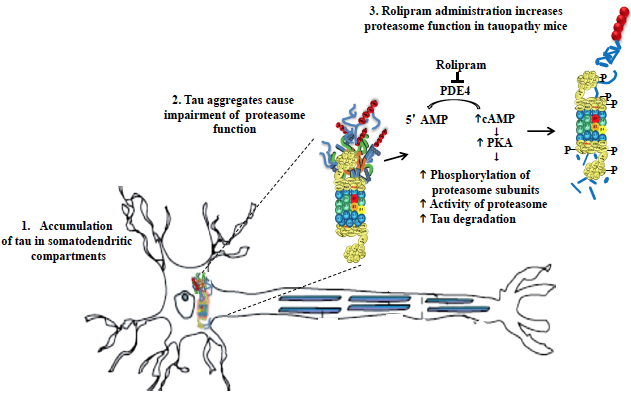 Tau-driven 26S proteasome impairment and cognitive dysfunction can be prevented early in disease by activating cAMP-PKA signaling.
|
To identify a druggable mechanism that could rescue proteasome function and reduce tau toxicity, we administered Rolipram, a PDE4 inhibitor, to mice with tauopathy hoping to prevent or reverse disease symptoms. Rolipram promotes activation of cAMP/PKA pathway, which had previously been shown by other Taub investigators (namely, Drs. Michael Shelanski and Ottavio Arancio) to improve proteasome mediated clearance. Building on these findings, and in collaboration with Prof. Alfred Goldberg at Harvard Medical School, we identified that cAMP/PKA activation phosphorylates proteasome subunits leading to increased activity of brain proteasomes, promoting removal of abnormal tau, and improving memory in mice with tauopathy.
Rolipram is a drug that is widely used in research and a good tool to study the general efficacy of this type of approach. However, there are similar (PDE4 inhibitors) drugs that are safer to use in patients, which can potentially have the same therapeutic effect on diseases such as AD, FTD, PD, etc. We are currently investigating the effects of different PDE inhibitors in collaboration with industry colleagues. More recently, we have started exploring the possibility of using G protein-coupled receptors (GPCRs), drugs that can enhance proteasome function in a similar way to rolipram. These drugs represent ~ 27% of all FDA approved drugs. Additionally, we aim to identify previously overlooked FDA approved drugs that work through this pathway that, because they are already FDA approved, might be used in patients very quickly. In general, a major goal of our lab is to use our insight, expertise, and biological resources (cell and mouse models) to identify and develop new therapies for neurodegenerative diseases, especially the tau-based diseases such as AD and FTD.
Natura Myeku, PhD
Associate Research Scientist in the Taub Institute
nm2631@columbia.edu
Karen Duff, PhD
Deputy Director, Taub Institute
Professor of Pathology and Cell Biology (in Psychiatry and in the Taub Institute)
ked2115@columbia.edu
 |  | |
| Deirdre O'Shea, MS | Yaakov Stern, PhD |
The relationship between subjective memory reports and objective memory performance in older adults is not clearly understood. Some studies have found that subjective memory is an accurate reflection of memory performance while other studies have not. Furthermore, previous studies have shown that depressive symptoms are positively associated with poor ratings of memory which may contribute to poor memory performance. Thus, identifying factors that mitigate the influence of depressive symptoms on cognitive performance in the face of perceived cognitive change in older adults is of key importance.
In this study by O'Shea et al. recently published in the International Journal of Geriatric Psychiatry, we examined whether 1) a well-established resilience factor perceived self-efficacy would attenuate the association between poor subjective memory and depressive symptoms. In addition, we explored whether greater self-efficacy would moderate the association between depressive symptoms and memory performances in a sub-sample of 1,196 adults with poor subjective memory ratings.
For the present study, data from 3,766 adults aged 65-90 were selected from the 2012 wave of the Health and Retirement Study. All analyses were adjusted for age, education, race/ethnicity, and gender. The results showed that poor ratings of memory were associated with more depressive symptoms and worse memory performance compared to more positive ratings of memory. The findings also showed that greater perceived self-efficacy attenuated the relationship between subjective memory and depressive symptoms. Furthermore, in the subsample of adults reporting poor subjective memory, those that performed the best had the highest levels of self-efficacy and lowest levels of depressive symptoms.
Together, these findings suggest 1) a role for depressive symptoms in the subjective-objective memory association and 2) that higher self-efficacy may buffer against the impact of poor subjective memory on one's mood and thereby attenuate the effect of depressive symptoms on objective memory performance. These results provide support for interventions that aim to enhance perceived self-efficacy in depressed older adults with memory complaints. However, this was a cross-sectional study so the direction of the reported associations can only be suggested. Future longitudinal studies would enhance our current understanding of the complex associations between mood, self-efficacy, and memory in the context of subjective memory reports.
Deirdre O'Shea, MS
Graduate Student
dmo2123@ufl.edu
Yaakov Stern, PhD
Professor of Neuropsychology
ys11@cumc.columbia.edu

