Columbia University
Irving Medical Center
Neurological Institute
710 West 168th Street, 3rd floor
(212) 305-1818
TaubCONNECT Research Perspectives:
November 2020
2: » APOE Ī4 and Resting-State Functional Connectivity in Racially/Ethnically Diverse Older Adults
3: » Assessment of Leisure Time Physical Activity and Brain Health in a Multiethnic Cohort of Older Adults
4: » Alzheimer-Related Altered White Matter Microstructural Integrity in Down Syndrome: a Model for Sporadic AD?
1: »
Chemogenetic attenuation of neuronal activity in the entorhinal cortex reduces AĪ² and tau pathology in the hippocampus
Alzheimer-Related Cerebrovascular Disease in Down Syndrome
1: »
Endothelial Activation of Caspase-9 Promotes Neurovascular Injury in Retinal Vein Occlusion
Tau is not Necessary for Amyloid-Beta-Induced Synaptic and Memory Impairments
IL-27: An Endogenous Constitutive Repressor of Human Monocytes
"Everything Hurts!" Distress in Semantic Variant Primary Progressive Aphasia
Down Syndrome: Distribution of Brain Amyloid in Mild Cognitive Impairment
Cortical Thickness and its Associations with Age, Total Cognition and Education Across the Adult Lifespan
Structural Brain Changes in Pre-Clinical FTD MAPT Mutation Carriers
Phospholipase D1 Ablation Disrupts Mouse Longitudinal Hippocampal Axis Organization and Functioning
Posttranslational Modifications Mediate the Structural Diversity of Tauopathy Strains
Profilin 1 Delivery Tunes Cytoskeletal Dynamics Toward CNS Axon Regeneration
Human Herpesvirus 6 Detection in Alzheimer's Disease Cases and Controls Across Multiple Cohorts
APOE4 is Associated with Differential Regional Vulnerability to Bioenergetic Deficits in Aged APOE Mice
Microglial Activation, but not Tau Pathology, is Independently Associated with Amyloid Positivity and Memory Impairment
CRISPR/Cas9 Editing of APP C-Terminus Attenuates β-Cleavage and Promotes α-Cleavage
Activity-Dependent Nucleation of Dynamic Microtubules at Presynaptic Boutons Controls Neurotransmission
Role of Tau Protein in Remodeling of Circadian Neuronal Circuits and Sleep
» Pum2 Shapes the Transcriptome in Developing Axons through Retention of Target mRNAs in the Cell Body
» Promotion of Axon Growth by the Secreted End of a Transcription Factor
» Increased Diameters of the Internal Cerebral Veins and the Basal Veins of Rosenthal Are Associated with White Matter Hyperintensity Volume
» Neuroinflammation in Frontotemporal Lobar Degeneration Revealed by 11CāPBR28 PET
» Live Imaging of ESCRT Proteins in Microfluidically Isolated Hippocampal Axons
» Alzheimer's Association International Conference (AAIC 2019)
» #1 Association of Variants in PINX1 and TREM2 With Late-Onset Alzheimer Disease
» #2 Brain Biomarkers and Cognition Across Adulthood
» #2 Brain Arterial Dilatation and the Risk of Alzheimer's Disease
» #2 FDG-PET Patterns Associated with Underlying Pathology in Corticobasal Syndrome
» #1 Effect of Aerobic Exercise on Cognition in Younger Adults: A Randomized Clinical Trial
» #1 A Tau Homeostasis Signature Is Linked with the Cellular and Regional Vulnerability of Excitatory Neurons to Tau Pathology
» #2 Between-network Functional Connectivity Is Modified by Age and Cognitive Task Domain
» #2 Semantic Network Function Captured by Word Frequency in Nondemented APOE Īµ4 Carriers
» First Place: NSUN2 is Dysregulated in Alzheimer's Disease
» #1 Homeostatic Plasticity Scales Dendritic Spine Volumes and Changes the Threshold and Specificity of Hebbian Plasticity
» #2 An MRI Measure of Degenerative and Cerebrovascular Pathology in Alzheimer Disease
» #1 A Multi-Omic Atlas of the Human Frontal Cortex for Aging and Alzheimer's Disease Research
» #2 Whole-exome Sequencing in 20,197 Persons for Rare Variants in Alzheimer's Disease
» #2 Preparation of Tau Oligomers After the Protein Extraction from Bacteria and Brain Cortices
» #2 Medical Retirement from Sport after Concussions: A Practical Guide for a Difficult Discussion
» #1 Cross Domain Self-Monitoring in Anosognosia for Memory Loss in Alzheimer's Disease
» #2 White Matter Changes in Alzheimer's Disease: A Focus on Myelin and Oligodendrocytes
» #1 ZCCHC17 is a Master Regulator of Synaptic Gene Expression in Alzheimer's Disease
» #2 Imaging Translocator Protein as a Biomarker of Neuroinflammation in Dementia
» #3 A Transcriptomic Atlas of Aged Human Microglia
» #1 Neuronal Lysosomal Dysfunction Releases Exosomes Harboring APP C-terminal Fragments and Unique Lipid Signatures
Association of APOE E2 Genotype with Alzheimer's and Non-Alzheimer's Neurodegenerative Pathologies
 |  |  | ||
| Terry E. Goldberg, PhD | Edward D. Huey, MD | Davangere P. Devanand, MBBS, MD |
The apolipoprotein E (APOE) gene contains both the major common risk variant for late onset Alzheimerās disease (AD), e4, and the major neuroprotective variant, e2. As recently published in Nature Communications, Drs. Terry Goldberg (Psychiatry and Anesthesiology), Ted Huey, and Davangere Devanand examined the association of APOE e2 with multiple neurodegenerative pathologies, leveraging the NACC v. 10 database of 1557 brains that included 130 e2 carriers and 679 e4 carriers, in order to examine potential neuroprotective effects.
For AD-related pathologies of amyloid plaques and Braak stage, e2 had large and highly significant protective effects contrasted with e3/e3 and e4 carriers with odds ratios of about 0.50 for e3 contrasts and 0.10 for e4 contrasts. In a mediation analysis, e2 had both direct and indirect effects on tau Braak stage. The indirect effect was via amyloid neuritic plaque burden and was larger than the direct effect. Thus, e2 might modulate spread of tangles through two processes. When they separately examined e2/e4 carriers, risk for AD pathologies was similar to that of e3/e4 carriers, not e2 carriers. Thus, in multiple analyses they found that e2/e4 was associated with greater degrees of Montine āABCā AD pathology in contrast to e2/e3 and e3/3 genotype, and could not be distinguished from e3/e4 cases with respect to amyloid or tau pathologies. Copy number was controlled, increasing the rigor of the approach. These results indicate that the e4 isoformās effects were neither blunted nor otherwise modified by e2 within the same brain, at least insofar as levels of the isoforms are in physiological range. This may have implications for trials in which the e2 isoform is introduced into the brains of e4 carriers in vivo.
E2 was not protective with respect to Lewy body pathology. Rather, APOE e4 increased risk for spread of Lewy bodies to limbic and neocortical regions but not for midbrain-only pathology. This result may suggest that while e4 does not increase risk for Parkinsonās disease, it may increase risk for Lewy body dementia.
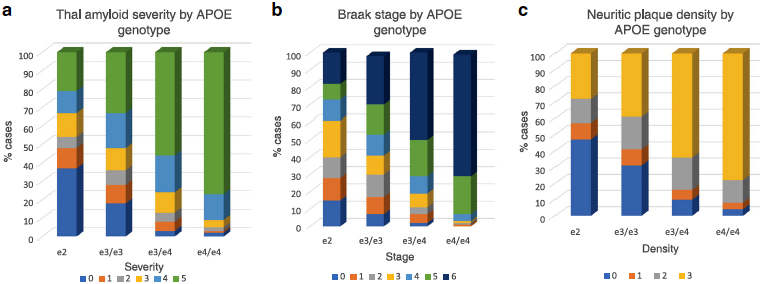 Figure 1: a Association of APOE genotype and diffuse amyloid plaque distribution. Within each APOE genotype column, colored rows represent the relative proportion of cases in each severity stage. These proportions are expressed as percentages and add to 100. There are increasing proportions of the most severe pathology (plaque stage 5) from the e2 to e4/e4 genotype groups in stepwise fashion. Amyloid stage 0 = no pathology; stage 5ā=āsevere. b Association of APOE genotype and Braak stage. Within each APOE genotype column, colored rows represent the relative proportion of cases in each severity stage. These proportions are expressed as percentages and add to 100. There are increasing proportions of the most severe pathology (Braak stages v and vi) from the e2 to e4/e4 genotype groups in stepwise fashion. Braak stage 0ā=āno pathology; stage viā=āsevere neocortical pathology. c Association of APOE genotype and neuritic amyloid plaque density level. Within each APOE genotype column, colored rows represent the relative proportion of cases in each severity stage. These proportions are expressed as percentages and add to 100. There are increasing proportions of the most severe pathology (plaque stages v and vi) from the e2 to e4/e4 genotype groups in stepwise fashion. Neuritic plaque stage 0ā=āno pathology; stage 3ā=āsevere pathology.
|
E2 associations with multiple frontotemporal lobar degeneration (FTLD) pathologies and tauopathies were complex. Initially, nominally albeit marginally significant associations with e2 were found, such that e2 promoted risk for Pick bodies, PSP, and TDP-43. However, when adjustments for AD histopathology were made, these findings were no longer significant.
Last, in a follow up study now published in the Journal of Neurology, Neurosurgery & Psychiatry, Goldberg et al. examined the associations of e2 with cerebrovascular disease in this sample. They did not find that e2 was associated with protection from CAA, infarcts, microinfarcts, and microhemorrhages. It was, however, associated with increased risk of gross hemorrhage (i.e., lobar hemorrhage).
The current study had several strengths. It is perhaps the largest single study of e2 and e4 effects on multiple post-mortem human neuropathologies. Findings were independent of clinical diagnosis; thus the authors did not impose any filter or a priori bias on analyses. In terms of design the study can be considered clinically transdiagnostic, as Goldberg et al. intentionally did not examine or otherwise adjust for clinical variables. Rather, they chose to focus on gold standard neuropathological diagnoses. Finally, NACC data were collected prospectively and without regard for the specific hypotheses that were tested. Analyses were rigorous as a study wide Bonferroni correction was used and all ordinal and logistic regressions adjusted for age of death and sex so that APOE's contribution to pathology could be examined independent of these factors. However, the case series had an ascertainment bias in that all cases came from Alzheimerās Centers and, not surprisingly, approximately 51% met pathological criteria for high AD neuropathological change scores by ABC system. In sum, Goldberg et al. found that e2 was associated with large, but circumscribed protective effects.
Terry E. Goldberg, PhD
Professor of Medical Psychology (in Psychiatry and Anesthesiology) at the Columbia University Medical Center
teg2117@cumc.columbia.edu
Edward D. Huey, MD
Associate Professor of Psychiatry and Neurology (in the Taub Institute for Research on Alzheimer's Disease and the Aging Brain)
edh2126@cumc.columbia.edu
Davangere P. Devanand, MBBS, MD
Professor of Psychiatry (in Neurology and in the Gertrude H. Sergievsky Center) at the Columbia University Medical Center
dpd3@cumc.columbia.edu
APOE Ī4 and Resting-State Functional Connectivity in Racially/Ethnically Diverse Older Adults
 |  | |
| Indira C. Turney, PhD | Adam M. Brickman, PhD |
Though the APOE Īµ4 allele is the most well-established genetic risk factor for Alzheimerās Disease (AD), studies show a weak association of APOE Īµ4 and AD risk among minority populations. Resting-state functional connectivity (rsFC), specifically within the default mode network (DMN), is vulnerable to the earliest stages of AD pathology, which accumulates years before symptom onset. Numerous neuroimaging studies demonstrated an association between APOE Īµ4 and rsFC of regions within the DMN, both in healthy populations and patients with AD. Nevertheless, it remains unclear whether the APOE Īµ4 allele differentially affects the brainās functional network architecture across race/ethnicity.
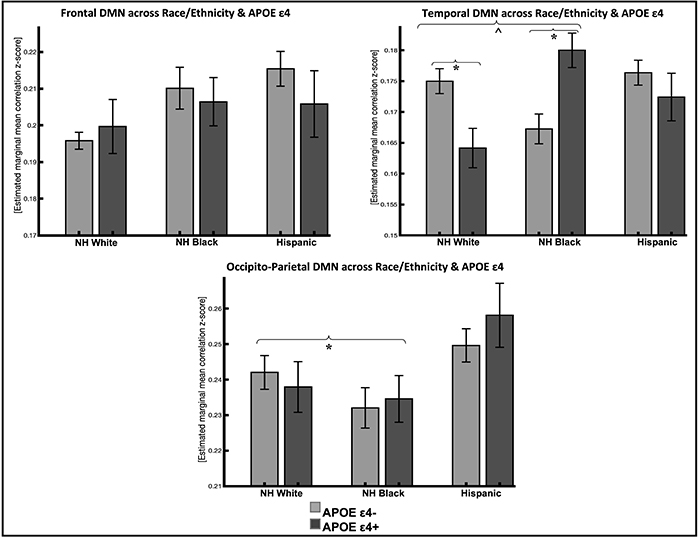 Figure 1: Resting-state functional connectivity in DMN subsystems across race/ethnicity and APOE e4 status (95% confidence interval error bars). Abbreviations: NH, non-Hispanic; DMN, default mode network; APOE, apolipoprotein E.
*P<.016 Bonferroni corrected; ĖP<.05 uncorrected
|
A recent study from the laboratory of Dr. Adam Brickman, including postdoctoral research scientist and first author Dr. Indira Turney, investigated the association between rsFC within the DMN and APOE Īµ4 across non-Hispanic whites, non-Hispanic Blacks, and Hispanics from the Washington Heights-Inwood Columbia Aging Project. As recently published in Alzheimer's and Dementia, they found that APOE Īµ4 carriers had lower rsFC in the temporal DMN subsystem, but only in non-Hispanic whites (Figure 1). Their findings suggest that APOE Īµ4 modulates rsFC in DMN subsystems differently in non-Hispanic whites compared to non-Hispanic Blacks and Hispanics. It is still critical that future studies examine how race and ethnicity modifies the risk and expression of AD to understand race-dependent biological mechanisms of AD.
Indira C. Turney, PhD
Postdoctoral Research Scientist in the Gertrude H. Sergievsky Center
ict2105@cumc.columbia.edu
Adam Brickman, PhD
Professor of Neuropsychology (in Neurology, the Taub Institute for Research on Alzheimer's Disease and the Aging Brain and the Gertrude H. Sergievsky Center)
amb2139@cumc.columbia.edu
 |  | |
| Yian Gu, MD, MS, PhD | Adam M. Brickman, PhD |
Results from longitudinal epidemiological studies, including a study published by Dr. Yian Gu and colleagues last year, suggest that regular leisure time physical activity (LTPA) is associated with reduced risk of dementia or Alzheimerās disease (AD). Multiple brain structural changes, including both neurodegeneration, such as volume loss, and cerebrovascular lesions, such as white matter hyperintensities, are powerful predictors for subsequent AD development. However, data on the association between LTPA and brain magnetic resonance imaging (MRI) measures remain scarce and inconsistent.
In a study recently published in JAMA Network Open, Drs. Yian Gu, Adam Brickman, and colleagues sought to examine the association of LTPA and MRI-assessed brain measures among multiethnic participants of the community based Washington/Hamilton Heights-Inwood Columbia Aging Project study.
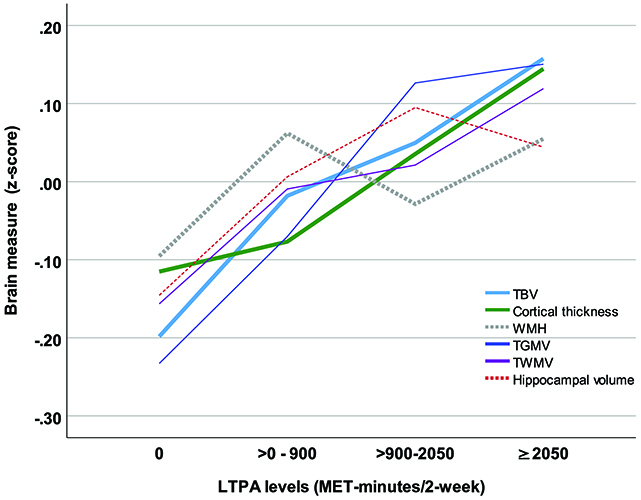 Figure 1: Brain measures by leisure time physical activity levels. Solid lines represent significant associations between leisure time physical activity (LTPA) and brain measures, while dotted lines indicate nonsignificant associations. TBV (total brain volume), TGMV (total gray matter volume), TWMV (total white matter volume), WMH (white matter hyperintensity).
|
The study found that, compared with nonactive older adults, those with the most LTPA had larger total brain volume (i.e., less brain atrophy), equivalent to approximately 3 to 4 years of healthy aging. A dose-response association was also found and even the lowest active LTPA level had benefits compared with the nonactive group (Figure 1). The association between LTPA and total brain volume was moderated by race/ethnicity, sex, and APOE status, but generally existed in all subgroups. The results remained similar after excluding participants with mild cognitive impairment. The study provides important evidence to suggest that more physical activity is helpful in maintaining brain health in older adults.
Yian Gu, MD, MS, PhD
Assistant Professor (in the Department of Neurology, Department of Epidemiology, and Taub Institute)
yg2121@cumc.columbia.edu
Adam Brickman, PhD
Professor of Neuropsychology (in Neurology, the Taub Institute for Research on Alzheimer's Disease and the Aging Brain and the Gertrude H. Sergievsky Center)
amb2139@cumc.columbia.edu
 |  | |
| H. Diana Rosas, PhD | Nicole Schupf, PhD |
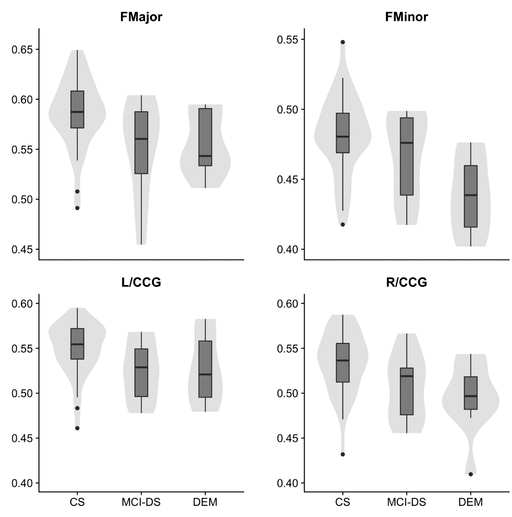
Figure 1: Violin and box plots describing the distribution of fractional anisotropy by diagnostic group. Fractional anisotropy values among mild cognitive impairmentāDown syndrome subjects were significantly lower in the forceps major, forceps minor, and left/right cingulumācingulate gyrus bundle compared to cognitively stable subjects
Virtually all adults with Down syndrome (DS) develop Alzheimerās disease (AD) associated neuropathology by the age of 40 and most will develop dementia by age 60. Several recent studies in sporadic AD have suggested that decreased regional white matter (WM) integrity is correlated with cognitive dysfunction. Other studies suggest that these changes may confer a particular susceptibility to dementia in individuals with DS. The latest study from Dr. Nicole Schupf and colleagues, including lead author Dr. H. Diana Rosas (Harvard), evaluated the relationship between altered WM integrity of tracts projecting to cortical areas involved in memory function, amyloid deposition, and cognitive function.
They found that early changes in late-myelinating and relative sparing of early-myelinating pathways were associated with regional amyloid deposition and cognitive deficits. Their findings, now published in Alzheimerās & Dementia: Diagnosis, Assessment & Disease Monitoring, suggest an important pathophysiological link between WM damage, amyloid deposition and clinical status prior to onset of dementia. Further, according to Ross et al., these findings āsupport the use of diffusion weighted imaging as an important and clinically relevant biomarker of progression of AD in individuals with DS, and may serve as a model for sporadic AD.ā
Nicole Schupf, PhD
Professor of Epidemiology (in Neurology, Psychiatry, the Gertrude H. Sergievsky Center, and the Taub Institute for Research on Alzheimer's Disease and the Aging Brain)
ns24@cumc.columbia.edu

