Columbia University
Irving Medical Center
Neurological Institute
710 West 168th Street, 3rd floor
(212) 305-1818
TaubCONNECT Research Perspectives:
July 2018
2: » Whole-exome Sequencing in 20,197 Persons for Rare Variants in Alzheimer's Disease
 |  | |
| Carlo Corona, PhD | Michael Shelanski, MD, PhD |
Normal cognitive function relies on the balance of neuronal excitability properties throughout the brain, as well as on synaptic plasticity. Among the many proteins reported to influence cognition, mounting evidence suggests a pivotal role for Activating Transcription Factor 4 (ATF4), a ubiquitously expressed member of the ATF/CREB transcription factor family of basic leucine zipper proteins. ATF4 has been postulated as a key regulator of learning and memory. The laboratory of Dr. Michael Shelanski previously reported that specific hippocampal ATF4 downregulation causes deficits in synaptic plasticity and memory and reduction of glutamatergic functionality. In their latest work, published recently in the Journal of Neuroscience, they extend their studies to address ATF4's role in neuronal excitability. Here, first author Carlo Corona and colleagues find that long-term ATF4 knockdown in cultured rat hippocampal neurons significantly increases the frequency of spontaneous action potentials. This effect is associated with decreased functionality of metabotropic GABAB receptors (GABABRs). Knocking down ATF4 results in significant reduction of GABABR-induced GIRK currents and increased mIPSC frequency. Furthermore, reducing ATF4 significantly decreases expression of membrane-exposed, but not total, GABABR 1a and 1b subunits, indicating that ATF4 regulates GABABR trafficking. In contrast, ATF4 knockdown has no effect on surface expression of GABABR2s, several GABABR-coupled ion channels or Ī²2 and Ī³2 GABAARs.
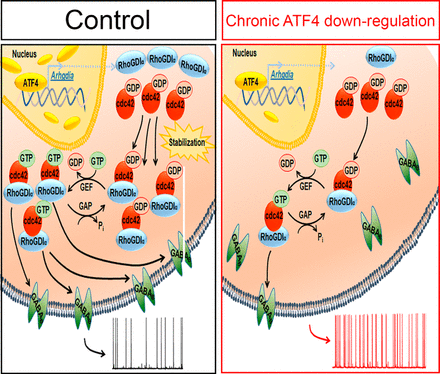
Figure: Proposed mechanism by which ATF4 regulates neuronal excitability. See full figure caption in JNeurosci.
Pharmacologic manipulations confirmed the relationship between GABABR functionality and action potential frequency in their cultures. Specifically, the effects of ATF4 downregulation cited above are fully rescued by transcriptionally active, but not by transcriptionally inactive, shRNA-resistant, ATF4. According to the authors, "We previously reported that ATF4 promotes stabilization of the actin-regulatory protein Cdc42 by a transcription-dependent mechanism. To test the hypothesis that this action underlies the mechanism by which ATF4 loss affects neuronal firing rates and GABABR trafficking, we downregulated Cdc42 and found that this phenocopies the effects of ATF4 knockdown on these properties. In conclusion, our data favor a model in which ATF4, by regulating Cdc42 expression, affects trafficking of GABABRs, which in turn modulates the excitability properties of neurons." Thus, their findings reveal a critical role for ATF4 in regulating the modulation of neuronal excitability by GABABRs.
Carlo Corona, PhD
Associate Research Scientist in the Department of Pathology and Cell Biology
cc3330@cumc.columbia.edu
Michael Shelanski, MD, PhD
Henry Taub Professor of Alzheimer's Disease and the Aging Brain (in Pathology and Cell Biology and the Taub Institute)
mls7@cumc.columbia.edu
Whole-exome Sequencing in 20,197 Persons for Rare Variants in Alzheimer's Disease
 |  | |
| Neha S. Raghavan, PhD | Richard Mayeux, MD, MSc |
The genetic bases of Alzheimer's disease (AD) remain uncertain. An international effort to fully articulate genetic risks and protective factors is underway, with the hope of identifying potential therapeutic targets and preventive strategies. Dr. Richard Mayeux and colleagues, including first author Neha Raghavan, used a gene based collapsing analysis developed by Dr. David Goldsteinās group in the Institute for Genomic Medicine (IGM) to identify and characterize the frequency and impact of ultra-rare variants in AD. They utilized whole exome sequencing data from 6,965 AD cases and 13,252 control individuals to identify loss of function, ultra-rare variants, with data from the Washington Heights-Inwood Columbia Aging Project, the Alzheimer's Disease Sequencing Project, and unrelated individuals from the IGM at Columbia University.
Their findings were published in Annals of Clinical and Translational Neurology earlier this month. This is the first investigation to establish a genome-wide, statistically significant association between multiple, extremely rare, loss of function variants in SORL1 and AD, in a large whole exome study of unrelated cases and controls. Of significance, of the 20 individuals that carried an extremely rare SORL1 loss of function variant, 19 were Alzheimerās disease cases, one had mild cognitive impairment, but no variants of this type were found in any of the controls. Also, age-at-onset was seven years earlier for patients with SORL1 qualifying variant compared with non-carriers. These findings further implicate ultra-rare, loss of function variants in SORL1 as a significant genetic risk factor for Alzheimer's disease, and provide a comprehensive dataset comparing the burden of rare variation in nearly all human genes in AD cases and controls.
Neha S. Raghavan, PhD
Postdoctoral Research Fellow in the Gertrude H. Sergievsky Center
nr2623@cumc.columbia.edu
Richard Mayeux, MD, MSc
Gertrude H. Sergievsky Professor of Neurology, Psychiatry and Epidemiology (in the Gertrude H. Sergievsky Center and in the Taub Institute)
rpm2@cumc.columbia.edu

