Columbia University
Irving Medical Center
Neurological Institute
710 West 168th Street, 3rd floor
(212) 305-1818
TaubCONNECT Research Perspectives:
February 2018
2: » Imaging Translocator Protein as a Biomarker of Neuroinflammation in Dementia
3: » A Transcriptomic Atlas of Aged Human Microglia
ZCCHC17 is a Master Regulator of Synaptic Gene Expression in Alzheimer's Disease
 |  |
 |
||
| Andrew F. Teich, MD, PhD | Zeljko Tomljanovic, MD | Mitesh Patel, MS |
Synaptic dysfunction is one of the earliest features of Alzheimer's disease (AD), and loss of synapses in post-mortem brain tissue correlates with pre-mortem cognitive status better than beta-amyloid or tau deposits (two defining hallmarks of AD). Published in Bioinformatics, Dr. Andrew Teich and colleagues define a set of transcriptional regulators that are computationally predicted to normally support the expression of synaptic genes, and whose dysfunction in AD is predicted to lead to loss of support for these genes and subsequent synaptic dysfunction. Transcriptional regulators that meet these criteria may be candidates for therapeutic intervention to rescue synaptic dysfunction in AD.
This list of candidate regulators was generated from human neuronal expression data using statistical associations between mRNA transcripts. In Tomljanovic et al., Teich and colleagues experimentally validate the top candidate from this effort, a protein called ZCCHC17. ZCCHC17 is a poorly studied protein that resides in the nucleus and may play a role in gene transcription. Teich and colleagues first show that ZCCHC17 is expressed in neurons and decreases early in AD, before significant neuronal loss or gliosis. They then knock-down ZCCHC17 in rat cortical neuronal cultures and show that the majority of ZCCHC17's target synaptic genes show the hypothesized decrease in expression. In addition, the ZCCHC17 human regulon (the total set of computationally derived gene targets) is predictive of equivalent interactions between ZCCHC17 and homologous genes in rat neurons, which supports a conserved function. Finally, the net effect on gene expression after ZCCHC17 knock-down preferentially affects ontology groups related to synaptic function, even when secondary changes in gene expression are included. Taken together, these data support the view that ZCCHC17 is a conserved nuclear protein whose loss early in AD contributes to loss of support for synaptic gene expression. The Teich laboratory is currently defining the physiologic consequences of ZCCHC17 loss in neurons, as well as the set of proteins that directly interact with ZCCHC17 in an attempt to better understand ZCCHC17's role in health and disease. Future work by the Teich laboratory will use the aforementioned computational tools to identify small molecules that can rescue synaptic dysfunction through modulation of disease-relevant transcriptional regulators, as well as to better understand drivers of autophagy and microglial dysfunction in AD.
Andrew F. Teich, MD, PhD
Assistant Professor of Pathology and Cell Biology (in the Taub Institute)
aft25@cumc.columbia.edu
Zeljko Tomljanovic, MD
Postdoctoral Research Scientist in the Department of Pathology and Cell Biology
zt2163@cumc.columbia.edu
Mitesh Patel, MS
Senior Research Technician at Memorial Sloan Kettering
mitesh_patel321@yahoo.com
Imaging Translocator Protein as a Biomarker of Neuroinflammation in Dementia

William C. Kreisl, MD
Neuroinflammation has long been considered a potential contributor to neurodegenerative disorders that result in dementia. Accumulation of abnormal protein aggregates in Alzheimer’s disease, frontotemporal dementia, and dementia with Lewy bodies is associated with the activation of microglia and astrocytes into proinflammatory states, and chronic low-level activation of glial cells likely contributes to the pathological changes observed in these and other neurodegenerative diseases. Most of Dr. William C. Kreisl’s career, thus far, has focused on the development of novel radioligands for the 18kDa translocator protein (TSPO)—a key biomarker for measuring inflammation in the brain via positron emission tomography (PET)—and translating use of these PET radioligands into clinical application in neurodegenerative diseases.
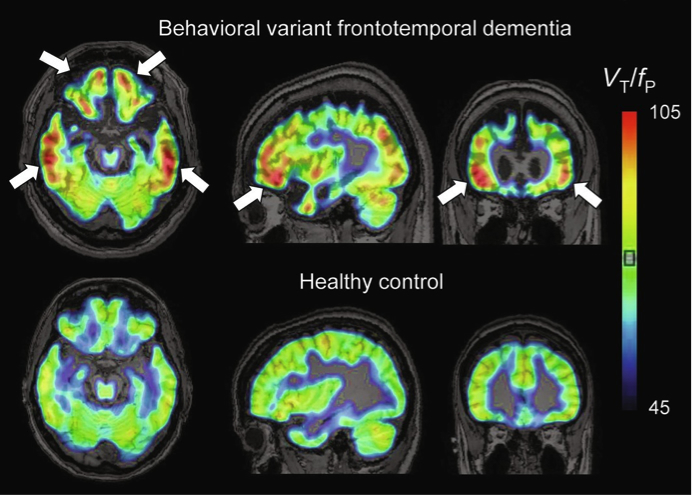
Figure: Representative11C-PBR28 parametric images (VT / fP) for a patient with behavioral variant FTD and a healthy control. Both subjects were high affinity binders (non-carriers of the rs6971 TSPO polymorphism). Arrows show increased binding in frontal and temporal cortices.
As reviewed by Dr. Kreisl and colleagues in the current volume of Advances in Pharmacology, increased TSPO density has been observed in brain tissue from patients with neurodegenerative diseases and colocalizes to activated microglia and reactive astrocytes. Several radioligands have been developed to measure TSPO density in vivo with PET, and these have been used in clinical studies of different dementia syndromes. However, TSPO radioligands have limitations, including low specific-to-nonspecific signal and differential affinity to a polymorphism on the TSPO gene, which must be taken into consideration in designing and interpreting human PET studies. Nonetheless, most PET studies have shown that increased TSPO binding is associated with various dementias, suggesting that TSPO has potential as a biomarker to further explore the role of neuroinflammation in dementia pathogenesis and may prove useful in monitoring disease progression. However, more information on the role of microglial activation and the causal relationships, if any, between microglial function and dementia pathogenesis is needed if TSPO PET is to be used to monitor response to immunomodulatory treatments.
William C. Kreisl, MD
Assistant Professor of Neurology (in the Taub Institute for Research on Alzheimer's Disease and the Aging Brain)
wck2107@cumc.columbia.edu
A Transcriptomic Atlas of Aged Human Microglia
 |  |  |
|||
| Elizabeth M. Bradshaw, PhD | Marta Olah, PhD | Philip L. De Jager, MD, PhD |
One focus of research for new Taub faculty members Drs. Elizabeth Bradshaw, Phil De Jager, and Marta Olah is the development of innovative approaches for investigating the fundamental role of microglia—the resident innate immune cells of the central nervous system (CNS)—in the pathogenesis of neurodegenerative diseases, such as Alzheimer's disease (AD), Parkinson's disease (PD), and multiple sclerosis (MS). According to the latest study led by Dr. Bradshaw, co-director for basic research in the new Center for Translational and Computational Neuroimmuniology (CTCN), the lack of a transcriptome-wide reference profile of human microglia from aged individuals limits our understanding of the aged microglia phenotype and function in humans, particularly in advanced age. Thus, the CTCN team sought to perform a comprehensive assessment of the transcriptomic landscape of aged human microglia and to create a resource for the community of neurodegenerative disease investigators.
Published in Nature Communications, Olah et al. present a novel protocol for the isolation of microglia from post mortem brain samples and an RNA-Seq library construction approach to create transcriptomic profiles from autopsy-derived microglia using only 5000 cells per individual. They establish the HuMi_Aged gene set that consists of genes primarily expressed in microglia in the aged human brain. This gene set displays many unique functional aspects that are highly relevant to brain aging. Furthermore, they show that this gene set is associated with neuropathological traits and susceptibility genes of AD, confirming the involvement of this cell type in the pathophysiology of the aging brain. Given that current transcriptomic approaches require hundreds of thousands of microglia cells, and the availability of fresh autopsy samples from well-characterized subjects is very limited, the protocol presented here will be a useful tool for the scientific community. In addition, the CTCN team provide the scientific community with the first transcriptomic atlas of aged human microglia (publicly available at http://shiny.maths.usyd.edu.au/Ellis/MicrogliaPlots).
Elizabeth M. Bradshaw, PhD
Adler Assistant Professor of Neurology (in Neurology, the Taub Institute for Research on Alzheimer's Disease and the Aging Brain and the Institute for Genomic Medicine)
emb2280@cumc.columbia.edu
Marta Olah, PhD
Instructor in Neurology at the Columbia University Medical Center
mo2738@cumc.columbia.edu
Philip L. De Jager, MD, PhD
Weil-Granat Professor of Neurology (in Neurology, the Taub Institute for Research on Alzheimer's Disease and the Aging Brain and the Precision Medicine Initiative)
pld2115@cumc.columbia.edu

