Columbia University
Irving Medical Center
Neurological Institute
710 West 168th Street, 3rd floor
(212) 305-1818
TaubCONNECT Research Perspectives:
February 2020
2: » Exceptionally Low Likelihood of Alzheimer’s Dementia in APOE2 Homozygotes from a 5,000-Person Neuropathological Study
Posttranslational Modifications Mediate the Structural Diversity of Tauopathy Strains
 |
 |
| Tamta Arakhamia and Christina E. Lee | Anthony Fitzpatrick, PhD |
Tau aggregation into insoluble filaments is the defining pathological hallmark of tauopathies. However, it is not known what controls the formation and templated seeding of strain-specific structures associated with individual tauopathies. In the present study, the laboratory of Taub faculty member Dr. Anthony Fitzpatrick, including research assistants and co-first authors Tamta Arakhamia and Christina E. Lee, along with colleagues from Mayo Clinic Florida, Emory, and Arizona State, used cryo-electron microscopy (cryo-EM) to determine the structures of tau filaments from corticobasal degeneration (CBD) human brain tissue. Published in the current issue of Cell, Cryo-EM and mass spectrometry of tau filaments from CBD reveal that this conformer is heavily decorated with posttranslational modifications (PTMs), enabling the investigators to map PTMs directly onto the structures. By comparing the structures and PTMs of tau filaments from CBD and Alzheimer’s disease, it is found that ubiquitination of tau can mediate inter-protofilament interfaces. Here, Fitzpatrick and colleagues propose a structure-based model in which cross-talk between PTMs influences tau filament structure, contributing to the structural diversity of tauopathy strains (Figure 1). Their approach establishes a framework for further elucidating the relationship between the structures of polymorphic fibrils, including their PTMs, and neurodegenerative disease.
|
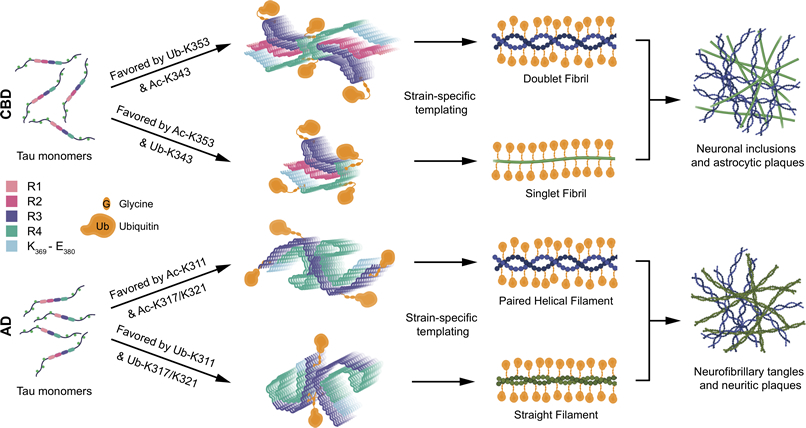
Figure 1: Proposed Structure-Based Model of How Interplay between PTMs Influences Tau Filament Structure Based on our cryo-EM maps and MS PTM mapping onto atomic models, we conclude that ubiquitination of tau can mediate inter-protofilament interfaces in the doublet CBD fibril and straight filament from AD. If sites on tau favoring the formation of doublet fibrils in CBD (K353) or straight filaments in AD (K311 and K317/321) are acetylated, or ubiquitinated with low occupancy, this inter-protofilament interface is less likely to form. The singlet fibril in CBD does not bind to a second protofilament, and paired helical filaments in AD have structures that do not require mediation by non-tau components at their inter-protofilament interface. The outcome of this model is that the incorporation of ubiquitin into tau filaments in CBD and AD mediates inter-protofilament packing resulting in distinct ultrastructural polymorphs, tuning the ratio of fibril subtypes in tau inclusions.
|
Anthony Fitzpatrick, PhD
Assistant Professor of Biochemistry and Molecular Biophysics
awf2130@cumc.columbia.edu
 |  |  |
|||
| Jean Paul G. Vonsattel, MD | Philip L. De Jager, MD, PhD | Richard Mayeux, MD, MSc |
Apolipoprotein E (APOE), the major susceptibility gene for late-onset AD, has three common alleles (APOE2, 3, and 4), giving rise to six genotypes (APOE2/2, 2/3, 3/3, 2/4, 3/4, and 4/4). Each additional copy of the APOE4 allele is associated with a higher risk of AD and a younger mean age at onset, while the presence of one or two copies of the APOE2 allele is associated with a lower risk of AD and an older mean age at onset. It remains to be clarified whether APOE2 homozygotes have a lower odd than persons with the APOE2/3 genotype, which is a question investigators from the Alzheimer’s Disease Genetics Consortium (ADGC) recently sought to address in a study of an unusually large number of clinically and neuropathologically classified Alzheimer’s dementia cases and controls.
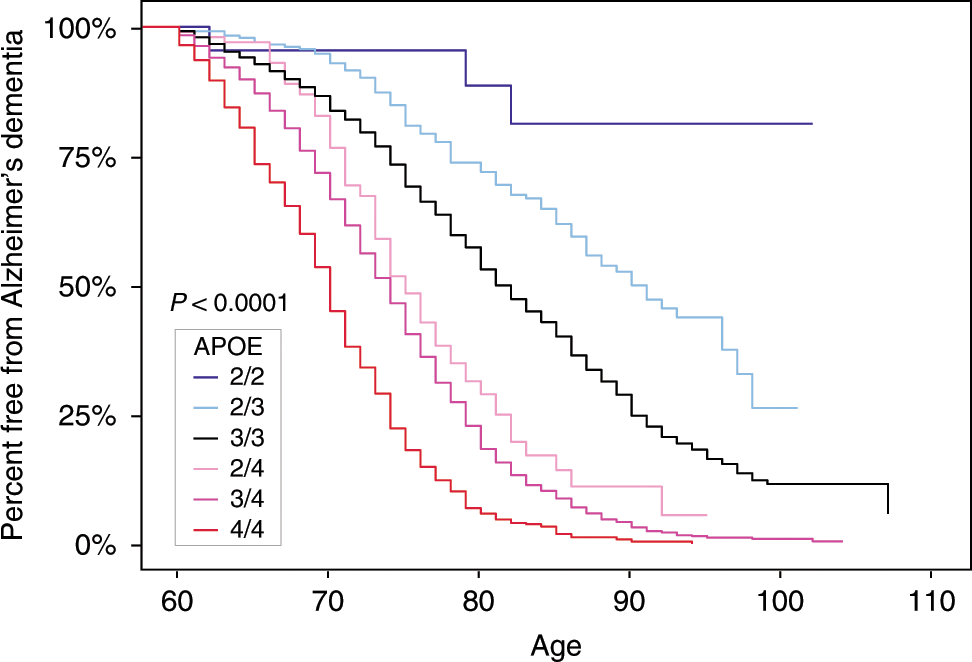
Capitalizing on data from more than 5,000 brain donors, the ADGC investigators, including Taub faculty members Drs. Jean Paul Vonsattel, Philip L. De Jager, and Richard P. Mayeux, found that APOE2/2 was associated with a low Alzheimer’s dementia odds ratios (ORs) compared to APOE2/3 and 3/3, and an exceptionally low OR compared to APOE4/4. Furthermore, the impact of APOE2 and APOE4 gene dose was significantly greater in the neuropathologically confirmed group than in more than 24,000 neuropathologically unconfirmed cases and controls. As published in the current issue of Nature Communications, this study provides updated ORs for Alzheimer’s dementia for each of the six common APOE genotypes, APOE2 allelic dose, and APOE4 allelic dose, on the differential risk of Alzheimer’s dementia, free from the confounding effects of clinically misdiagnosed cases and controls. More generally, according to the authors, it highlights the impact that “discovering and targeting the mechanism by which APOE variants account for differential risk could have on the understanding, treatment, and prevention of AD, including those interventions that might prevent both the initial development of AD pathology and the subsequent development of dementia.”
Jean Paul G. Vonsattel, MD
Professor of Pathology and Cell Biology at the CUMC
jgv2001@cumc.columbia.edu
Philip L. De Jager, MD, PhD
Weil-Granat Professor of Neurology (in Neurology, the Taub Institute for Research on Alzheimer's Disease and the Aging Brain and the Precision Medicine Initiative)
pld2115@cumc.columbia.edu
Richard Mayeux, MD, MSc
Gertrude H. Sergievsky Professor of Neurology, Psychiatry and Epidemiology (in the Gertrude H. Sergievsky Center and in the Taub Institute)
rpm2@cumc.columbia.edu