Columbia University
Irving Medical Center
Neurological Institute
710 West 168th Street, 3rd floor
(212) 305-1818
TaubCONNECT Research Perspectives:
October 2019
» 2: Promotion of Axon Growth by the Secreted End of a Transcription Factor
» Pum2 Shapes the Transcriptome in Developing Axons through Retention of Target mRNAs in the Cell Body
» Promotion of Axon Growth by the Secreted End of a Transcription Factor
» Increased Diameters of the Internal Cerebral Veins and the Basal Veins of Rosenthal Are Associated with White Matter Hyperintensity Volume
» Neuroinflammation in Frontotemporal Lobar Degeneration Revealed by 11CāPBR28 PET
» Live Imaging of ESCRT Proteins in Microfluidically Isolated Hippocampal Axons
» Alzheimer's Association International Conference (AAIC 2019)
» #1 Association of Variants in PINX1 and TREM2 With Late-Onset Alzheimer Disease
» #2 Brain Biomarkers and Cognition Across Adulthood
» #2 Brain Arterial Dilatation and the Risk of Alzheimer's Disease
» #2 FDG-PET Patterns Associated with Underlying Pathology in Corticobasal Syndrome
» #1 Effect of Aerobic Exercise on Cognition in Younger Adults: A Randomized Clinical Trial
» #1 A Tau Homeostasis Signature Is Linked with the Cellular and Regional Vulnerability of Excitatory Neurons to Tau Pathology
» #2 Between-network Functional Connectivity Is Modified by Age and Cognitive Task Domain
» #2 Semantic Network Function Captured by Word Frequency in Nondemented APOE Īµ4 Carriers
» First Place: NSUN2 is Dysregulated in Alzheimer's Disease
» #1 Homeostatic Plasticity Scales Dendritic Spine Volumes and Changes the Threshold and Specificity of Hebbian Plasticity
» #2 An MRI Measure of Degenerative and Cerebrovascular Pathology in Alzheimer Disease
» #1 A Multi-Omic Atlas of the Human Frontal Cortex for Aging and Alzheimer's Disease Research
» #2 Whole-exome Sequencing in 20,197 Persons for Rare Variants in Alzheimer's Disease
» #2 Preparation of Tau Oligomers After the Protein Extraction from Bacteria and Brain Cortices
» #2 Medical Retirement from Sport after Concussions: A Practical Guide for a Difficult Discussion
» #1 Cross Domain Self-Monitoring in Anosognosia for Memory Loss in Alzheimer's Disease
» #2 White Matter Changes in Alzheimer's Disease: A Focus on Myelin and Oligodendrocytes
» #1 ZCCHC17 is a Master Regulator of Synaptic Gene Expression in Alzheimer's Disease
» #2 Imaging Translocator Protein as a Biomarker of Neuroinflammation in Dementia
» #3 A Transcriptomic Atlas of Aged Human Microglia
» #1 Neuronal Lysosomal Dysfunction Releases Exosomes Harboring APP C-terminal Fragments and Unique Lipid Signatures
Pum2 Shapes the Transcriptome in Developing Axons through Retention of Target mRNAs in the Cell Body
 |
 |
| JosĆ© C. MartĆnez, MD, PhD | Ulrich Hengst, PhD |
Neurons are among the most morphologically complex cells in the body, containing extensive arborizations for receiving inputs from other neurons, and sending signals through long axons projecting up to one meter. A key mechanism underlying the development and maintenance of this complex structure is the localized production of a particular subset of proteins in distal projections. In order to accomplish this, neurons send mRNA transcripts serving as templates for these proteins into projections. These mRNAs can then be translated into protein on demand. The current model for how neurons accomplish this involves the active transport of mRNAs by RNA-binding proteins into projections, primarily through the recognition of specific sequences in mRNAs. While several such sequences have been identified in axonally localized mRNAs, the elements responsible for the localization of most axonal mRNAs remain unknown.
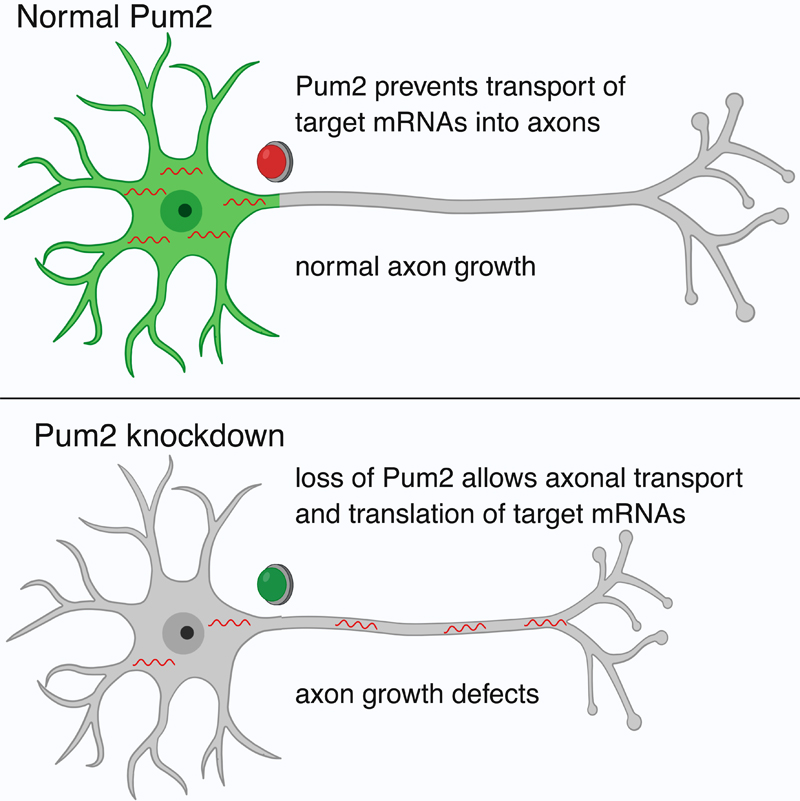
In a recent article published in Neuron, Dr. Ulrich Hengst and colleagues set out to discover novel mechanisms of axonal mRNA localization that might be shared among many mRNAs. The project, led by first author and former graduate student in the Hengst lab, Dr. JosĆ© MartĆnez, revealed that a short sequence of eight nucleotides known as the Pumilio binding element (PBE) is enriched in mRNAs that are localized to the soma of developing neurons, but depleted in mRNAs that are localized to axons. The PBE is recognized and bound by the RNA-binding proteins Pumilio (Pum) 1 and 2. Pum2 was found to be specifically localized to the soma, where it acts to prevent the axonal localization of its targets. Reduction of Pum2 or blockage of the PBE resulted in an increase in axonal localization of target mRNAs, while adding the PBE to axonal mRNAs was sufficient to decrease their axonal localization. Furthermore, Pum2 reduction resulted in a decrease in axonal growth and branching in the cortex, as well as impaired regeneration after injury in sensory neurons.
Together, MartĆnez et al. report a novel mechanism controlling the localization of mRNAs in developing neurons. In addition to previously reported active transport mechanisms, they find that mRNAs can also be localized by exclusion. Additionally, this regulatory relationship may play a role in axonal growth and branching, suggesting that it could be an important component of neural development.
Ulrich Hengst, PhD
Associate Professor of Pathology and Cell Biology (in the Taub Institute for Research on Alzheimer's Disease and the Aging Brain)
uh2112@cumc.columbia.edu
Promotion of Axon Growth by the Secreted End of a Transcription Factor
 |
 |
| Ethan McCurdy, PhD | Ulrich Hengst, PhD |
Developing neurons face the challenge of spanning immense distances to form synapses with their targets. During neural development, axons respond to a scant number of extracellular signaling molecules that help control aspects of axon growth. However, since the number of these molecules is limited, other types of control exerted at a local level are thought to regulate the extending axon. However, the extent to which axons can actively control their own development is unknown.
Recent work published in Cell Reports by Taub faculty member Ulrich Hengst and lead author Ethan McCurdy has identified a cell-intrinsic mechanism by which axons control their own growth through the generation of the secreted portion of a transcription factor. The authors found that the intramembranous protease S2P cleaves the ER stress-related transcription factor CREB3L2 in developing axons, leading to the secretion of its luminal domain. Upon secretion, this portion of CREB3L2 binds the chemoattractive morphogen Shh and promotes its association with the receptor Ptch1, promoting axon growth.
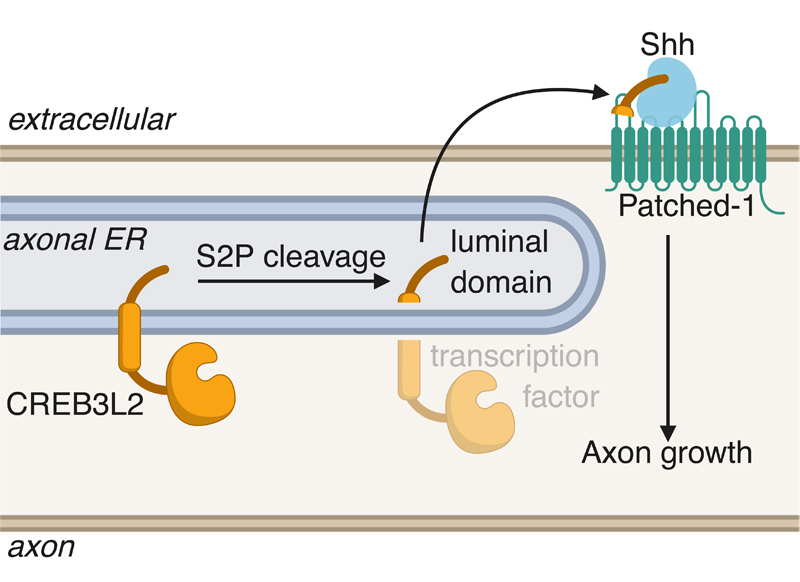
The significance of this project is the identification of a neuron-intrinsic mechanism for regulating axon growth, placing the axon in a position where it actively controls its own development. Several reports have come out over the last few years that highlight the unconventional activation of stress pathways during normal neuronal physiology. In McCurdy et al. 2019, the authors also note that since S2P and CREB3L2 are activated during the ER stress response, this observation suggests the importance of baseline levels of cellular stress for the developing nervous system.
Ulrich Hengst, PhD
Associate Professor of Pathology and Cell Biology (in the Taub Institute for Research on Alzheimer's Disease and the Aging Brain)
uh2112@columbia.edu

