Columbia University
Irving Medical Center
Neurological Institute
710 West 168th Street, 3rd floor
(212) 305-1818
TaubCONNECT Research Perspectives:
May 2018
2: » Oligomeric Aβ1-42 Triggers the Generation of a Retrograde Signaling Complex from Sentinel mRNAs in Axons
|
Family history of dementia is a major risk factor for LOAD (Mayeux et al., 1991; Lautenschlager et al., 1996). Studies in large pedigrees (Rogaeva et al., 2006) with early onset Alzheimerâs Disease (EOAD) identified APP, PSEN1, and PSEN2 genes (Goate et al., 1991; Sherrington et al., 1995; Levy-Lahad et al., 1995) as causal genes. These studies identified amyloid-β aggregation as the hallmark characteristic of LOAD. The largest twin study in an elderly Swedish cohort estimated heritability of LOAD to be between 58%-79% (Gatz et al., 2006). Twin studies have also shown heritability of LOAD is higher in monozygotic twins, and first degree relatives inherit two-fold expected lifetime risk of getting LOAD.
Large-scale sequencing efforts including the Alzheimerâs Disease Sequencing Project (ADSP) and its follow-up study (ADSP-FUS) are underway to identify rare causal variants in LOAD. These studies are designed to study extended families and a large number of cases and age-matched healthy elderly controls to identify rare-variants (RVs) associated with LOAD. Despite RVs conferring small population attributable risk, individuals carrying these RVs have substantially higher risk. Families with a large number of patients and aggregation of RVs represent a powerful approach to investigate rare-variant hypothesis in LOAD.
Dr. Badri Vardarajan and colleagues from Taub have previously shown that Caucasian families have a three-fold and Caribbean Hispanic families a five-fold higher incidence rate of LOAD compared to the general population (Vardarajan et al., 2014). Caribbean Hispanic (CH) families from the Dominican Republic represent a ârelativeâ population isolate with a limited number of founders, and evidence of inbreeding and the degree of inbreeding is a significant predictor of LOAD risk (Vardarajan et al., 2014).
With Dr. Sandra Barral, Dr. Vardarajan first performed linkage analyses in the 67 CH families of the ADSP discovery project (Barral et al., 2015) to identify regions that may contain disease causative variants. They identified 26 regions linked to LOAD (HLOD â¥3.6), and validated 13 of the regions (HLOD â¥2.5) using the entire family sample. The strongest signal was at 11q12.3 (rs2232932: HLODmax = 4.7, Pjoint = 6.6 à 10(-6)), a locus located â¼2 Mb upstream of the membrane-spanning 4A gene cluster. They additionally identified a locus at 7p14.3 (rs10255835: HLODmax = 4.9, P-joint = 1.2 à 10(-5)), a region harboring genes associated with the nervous system (GARS, GHRHR, and NEUROD6).
In the present study, published in the Annals of Clinical and Translational Neurology, Dr. Vardarajan and colleagues analyzed whole genome sequences (WGS) from 351 members of 67 CH families from the Dominican Republic and New York multiply affected by LOAD. These families plus an additional 47 Caucasian families underwent WGS as a part of the ADSP. All members of 67 CH families, an additional 48 CH families, and an independent CH case-control cohort were subsequently genotyped for validation. Patients met criteria for LOAD and controls were determined to be dementia free. Rare variants segregating within families and gene-based associations with disease within LOAD GWAS loci were investigated.
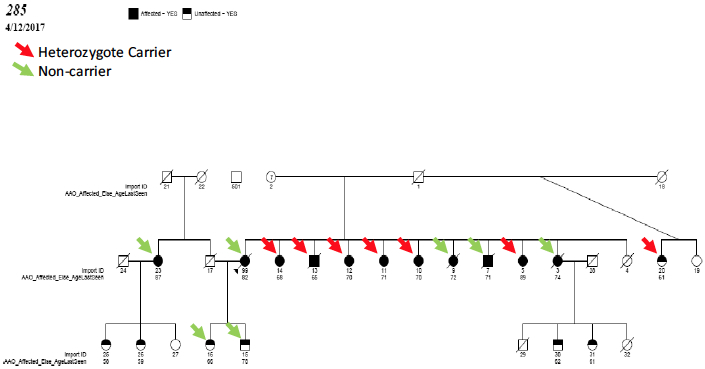 Figure 2. Segregation pattern of
AKAP9 p.R434W in family 285 (Refer to Table S8 for conversion for EFIGA to ADSP ids).
|
Vardarajan et al. found a variant in AKAP9, p.R434W, segregated two large families and was significantly associated (OR = 5.77, 95% CI: 1.07-30.9, P = 0.041). In addition, missense mutations in MYRF and ASRGL1 under previously reported linkage peaks at 7q14.3 and 11q12.3 segregated completely in one family and in follow-up genotyping both were nominally significant ( P < 0.05). They also identified rare variants in a number of genes associated with LOAD in prior genome wide association studies, including CR1 ( P = 0.049), BIN1 ( P = 0.0098) and SLC24A4 (P = 0.040).
These results suggest that rare variants in multiple genes influence the risk of LOAD disease in multiplex families, and that rare variants may underlie loci identified in genome wide association studies.
Badri N. Vardarajan, PhD, MS
Assistant Professor of Neurological Science (in Neurology, the Gertrude H. Sergievsky Center, and the Taub Institute)
bnv2103@cumc.columbia.edu

Ulrich Hengst, PhD
In Alzheimer's disease (AD) and related disorders, neurodegenerative stimuli act most frequently first on neurites and elicit local pathological changes. Additionally, neurodegenerative stimuli such as the AD peptide oligomeric Aβ1-42 trigger also neuron-wide changes. The intra-cellular spread of pathogenic changes from neurites to the neuronal cell bodies might contribute to the propagation of AD pathology through the brain, leading to the successive functional impairment of specific regions of the brain and consequently progressive cognitive decline. In 2014, the group of Dr. Hengst had shown that selective exposure of axons to oligomeric Aβ1-42 is sufficient to induce the degeneration of a neuron (Baleriola et al., 2014). A new study by the laboratory of Ulrich Hengst, published in EMBO reports, addressed the question which signaling mechanisms are rapidly activated within Aβ1-42-treated axons to relay the information concerning the presence of Aβ1-42 from the axon to the soma. Knowledge of the signaling pathways by which the information of the insult is transmitted to the cell body is crucial to understand disease progression in the brain characteristic for AD, and approaches to stop or slow down the spread could potentially interfere with disease progression and lengthen the lifespan of AD patients.
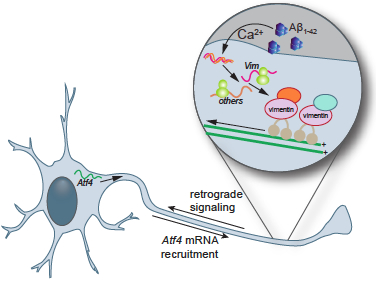
Figure: Aβ1-42 triggers the immediate translation of sentinel mRNAs including vimentin in axons whose protein products form signaling complexes that transmit the news of Aβ1-42's presence from the cellular periphery back to the neuronal soma.
First author Chandler Walker and her colleagues found that within 15 minutes of its local application to axons Aβ1-42 triggers the induction of a short-lived burst of protein synthesis. This induction of protein synthesis is accompanied by and requires an increase in the concentration of intra-cellular calcium. The burst in axonal protein synthesis produces the components of a retrograde signaling complex that is required to transmit to information regarding the presence of Aβ1-42 from the cellular periphery to the cell body. The authors identified a core component of this signaling complex: vimentin. Inhibition of vimentin synthesis in axons or of its transport to the cell bodies prevents the induction of pathological changes in the neuronal soma. Together, these findings reveal that axons in the brain contain sentinel mRNAs that are silenced under normal conditions but are translated into components of a retrograde signaling complex in response to local neurodegenerative stimuli.
This mechanism is reminiscent to the situation in injured axons of the peripheral nervous system. However, the identity of the retrograde signaling complexes differs in Aβ1-42-treated and injured axons. Thus, the principle âgeneration of a retrograde signaling complex by local translationâ appears to be a general response of neurons to injuries or degenerative stimuli that has been adopted to accurately relay the information regarding a variety of insults.
Ulrich Hengst, PhD
Associate Professor of Pathology and Cell Biology (in the Taub Institute for Research on Alzheimer's Disease and the Aging Brain
uh2112@cumc.columbia.edu



