Columbia University
Irving Medical Center
Neurological Institute
710 West 168th Street, 3rd floor
(212) 305-1818
TaubCONNECT Research Perspectives:
May 2020
2: » Metabolic Correlates of Prevalent Mild Cognitive Impairment and Alzheimer's Disease in Adults with Down Syndrome
3: » Down Syndrome: Distribution of Brain Amyloid in Mild Cognitive Impairment
"Everything Hurts!" Distress in Semantic Variant Primary Progressive Aphasia
 |  | |
| Megan S. Barker, PhD | Edward D. Huey, MD |
Frontotemporal dementia (FTD) is the most common dementia in people younger than 60, and approaches the prevalence of Alzheimer’s disease prior to age 65. FTD most commonly presents as a behavioral phenotype (bvFTD), which is a disorder primarily affecting behavior and personality. Less frequently, patients with FTD display a predominant degeneration of language, and in a subset of cases this manifests as a progressive degradation of semantic knowledge (i.e., understanding and recognizing the meaning of words and/or objects). This phenotype is known as semantic variant primary progressive aphasia (svPPA).
Like bvFTD, svPPA is associated with behavioral changes, and previous research has shown overlap in broad categories of behaviors (e.g. obsessions, compulsions). However, differences between the neuropsychiatric profiles of bvFTD and svPPA are largely unknown. Thus, Drs. Ted Huey and Stephanie Cosentino, together with postdoctoral fellow and first author Dr. Megan Barker and coauthors Hannah Silverman, Dr. Rachel Fremont and Masood Manoochehri, performed detailed chart reviews in order to document behavioral symptoms in a case series of patients with svPPA. A small group of bvFTD patients was included as a contrast group. The authors reported that although svPPA and bvFTD patients both exhibited repetitive behaviors and eating changes, these symptoms were associated with intense distress and dysphoria in svPPA patients, which was not observed in bvFTD. SvPPA patients also had somatic complaints and tactile sensitivity, with some patients describing near constant states of physical discomfort, stating that ‚Äúeverything hurts‚ÄĚ. Finally, unlike bvFTD patients, svPPA patients often presented with mood lability intermixed with irritability or hypomanic affect, and often endorsed suicidal ideation. Overall, as recently reported in Cortex, emotional distress appears to be an important aspect of the svPPA neuropsychiatric profile. In many ways this represents a mirror contrast to the emotional blunting characteristic of bvFTD.
Megan S. Barker, PhD
Postdoctoral Fellow in the Taub Institute for Research on Alzheimer's Disease and the Aging Brain
msb2228@cumc.columbia.edu
Edward D. Huey, MD
Associate Professor of Psychiatry and Neurology (in the Taub Institute for Research on Alzheimer's Disease and the Aging Brain)
edh2126@cumc.columbia.edu
 |  | |
| Nicole Schupf, PhD | Mark Mapstone, PhD |
People with Down syndrome are thought to have increased risk for Alzheimer’s disease due to triplication of the amyloid precursor protein encoded by APP on chromosome 21. However, the onset of clinical features of dementia is variable suggesting that other factors may serve to accelerate or slow the trajectory of the disease. In a recent manuscript published in Alzheimer’s and Dementia: Diagnosis and Disease Monitoring, Taub faculty member Dr. Nicole Schupf, and lead author Dr. Mark Mapstone of the University of California, Irvine report that specific metabolic factors assessed in peripheral blood may be linked to transitions to dementia in people with Down Syndrome and could serve as biomarkers of disease progression.
Schupf, Mapstone, and their colleagues collected peripheral blood from 292 participants enrolled in the Alzheimer’s Biomarker Consortium Down Syndrome (ABC-DS). Dr. Schupf is one of the PI’s of the ABC-DS and the Taub Institute is one of the principle sites. Thirty-eight participants met criteria for AD (DS-AD), 43 met criteria for mild cognitive impairment (DS-MCI), and 211 were cognitively unaffected and stable (CS). They used untargeted mass spectrometry to measure small molecular weight molecules and found a total of 180 metabolites that were differentially expressed among the three groups (FDR q < 0.05).
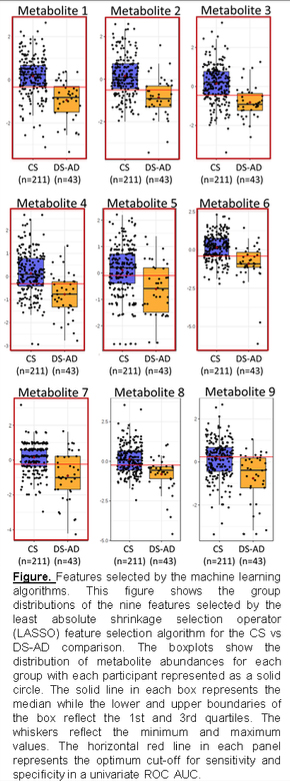
They examined the loadings of these metabolites on canonical metabolic pathways and found enrichment in fatty acid/lipid metabolism and central carbon/carbohydrate metabolism pathways associated with dementia progression with enrichment specifically for fatty acid metabolism between adjacent phenotypic states (e.g. CS vs DS-MCI and DS-MCI vs DS-AD).
The authors constructed classifier models using machine learning feature selection (see Figure) and found that the CS and DS-AD groups could be classified with a 9-metabolite model (ROC AUC = 0.87, sensitivity =0.84, specificity= 0.87) and the DS-MCI and DS-AD groups could be classified using a 5-metabolite model (ROC AUC = 0.89, sensitivity = 0.79, specificity = 0.86).
These findings are similar to metabolic alterations seen by other investigators studying late onset Alzheimer’s disease in the neurotypical population and suggest that changes in specific metabolic pathways may herald dementia phenoconversion events in people with Down syndrome.
Nicole Schupf, PhD
Professor of Epidemiology (in Neurology, Psychiatry, the Gertrude H. Sergievsky Center, and the Taub Institute for Research on Alzheimer's Disease and the Aging Brain)
ns24@cumc.columbia.edu
Mark Mapstone, PhD
Professor, Department of Neurology
University of California‚ÄźIrvine
mark.mapstone@uci.edu
Down Syndrome: Distribution of Brain Amyloid in Mild Cognitive Impairment
 |  |
|
| Nicole Schupf, PhD | David Keator, MS, PhD and study participant Lee Ann Diehl |
Studying adults with Down Syndrome (DS) provides valuable insight into the nature and progression of Alzheimer’s Disease (AD). The predominant genetic basis of DS is a triplication of chromosome 21 containing, among others, the amyloid precursor protein (APP). Interestingly, individuals with DS may be at a higher risk for developing AD compared to other individuals with intellectual disability, with the triplication and overexpression of APP playing a key role.
Interventions targeting Aő≤ plaques after the clinical onset of dementia have predominantly been unsuccessful, suggesting a need for early biomarkers predictive of conversion. Positron emission tomography (PET) has been used to measure Aő≤ in the brain through the development of tracers sensitive to Aő≤ plaques. The current study, co-authored by Drs. Nicole Schupf and Adam Brickman, with lead authors Drs. David Keator, Ira Lott, and colleagues from the University of California-Irvine, as part of the Alzheimer‚Äôs Biomarkers Consortium - Down Syndrome (ABC-DS), aimed to characterize regional brain amyloid in participants with mild cognitive impairment (MCI-DS), considered an early stage of clinical progression of AD preceding a diagnosis of dementia, compared to those that are cognitively stable (CS).
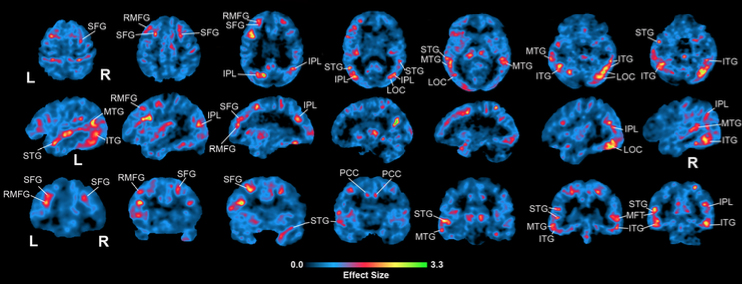 Figure 1: Voxel-based contrasts of MCI-DS vs. CS showing effect sizes of increased amyloid load in MCI-DS compared to CS. Areas of increase corresponding to a-priori hypothesized regions are labeled with abbreviations: IPL, inferior parietal lobe; ITG, inferior temporal gyrus; LOC, lateral occipital cortex; LOF, lateral orbitofrontal; MTG, middle temporal gyrus; RMFG, rostral middle frontal gyrus; PCC, posterior cingulate cortex; SFG, superior frontal gyrus; STG, superior temporal gyrus.
|
As published recently in Alzheimer’s and Dementia Diagnosis, Assessment, and Disease Monitoring, 18F-AV-45 (florbetapir) PET data was collected to evaluate the amyloid burden in 58 participants clinically classified as CS or MCI-DS and 12 longitudinal CS participants. The study confirmed their hypotheses of increased amyloid in MCI-DS compared to CS in apriori-hypothesized brain regions (Figure 1), highlighting the regional nature of amyloid load in the study groups. Regions showing the largest increase in amyloid load in MCI-DS include the inferior parietal and superior frontal regions (~24%), superior temporal and rostral middle frontal regions (~11% and 13%), and the dorsal striatum (~11%). Because dementia is a progressive illness, Keator et al. expected to see evidence of increasing amyloid in cognitively-stable participants evaluated longitudinally in the regions identified in our cross-sectional comparison between the MCI-DS and CS groups. Consistent with their cross-sectional analyses, they saw the largest annual increases in amyloid in rostral middle frontal and superior frontal regions of 4.1% increase per year, followed by regions of temporal lobe, cingulate, and striatum of ~3-3.5% per year.
Based on the consistency of their results and in light of prior studies, Keator et al. feel confident that the unique brain amyloid signatures discussed here are reliable indicators of AD progression in DS. Future work will consist of expanding and following their current sample longitudinally. They expect the rich longitudinal dataset will help further confirm their inferences, provide the opportunity to evaluate amyloid and tau signatures in MCI-DS, and evaluate the identification of composite biomarkers that predict transitions from cognitively stable to mild cognitive impairment and eventual dementia.
Nicole Schupf, PhD
Professor of Epidemiology (in Neurology, Psychiatry, the Gertrude H. Sergievsky Center, and the Taub Institute for Research on Alzheimer's Disease and the Aging Brain)
ns24@cumc.columbia.edu
David B. Keator, MS, PhD
Research Professor, Department of Psychiatry and Human Behavior
Operations Director, Neuroscience Imaging Center, University of California, Irvine
dbkeator@hs.uci.edu

