Columbia University
Irving Medical Center
Neurological Institute
710 West 168th Street, 3rd floor
(212) 305-1818
TaubCONNECT Research Perspectives:
October 2020
2: » Alzheimer-Related Cerebrovascular Disease in Down Syndrome
3: » Depression is Associated With Preserved Cortical Thickness Relative to Apathy in Frontotemporal Dementia
 |  | |
| Gustavo A. Rodriguez, PhD | Geoffrey M. Barrett | |

|  | |
| Karen Duff, PhD | Abid Hussaini, PhD |
The entorhinal cortex (EC) and hippocampus (HIPP) are two brain structures that play a critical role in spatial learning and memory. Importantly, the EC is one of the first brain structures to exhibit pathological tau accumulation and subsequent neuronal loss in Alzheimerâs disease (AD). As AD progresses, considerable accumulation of pathological tau continues from the EC into the HIPP in a stereotypical manner. However, the biological mechanisms underlying tau propagation are not fully understood.
The accumulation of amyloid beta (Aβ) in the brain, a hallmark of AD pathology, may trigger the aggregation and acceleration of tau pathology via an intermediate mechanism, without the need for direct Aβ-tau interaction. Previous reports demonstrate that increased neuronal activity leads to Aβ and tau release in vivo, and can exacerbate Aβ deposition and tauopathy in synaptically connected neurons.
Taub Institute investigators Drs. Abid Hussaini and Karen Duff (now Centre Director of UK Dementia Research, University College London) combined efforts in the lab to investigate the impact of pathology on EC neuronal activity in a newly created mouse that models both Aβ and tau pathology. Dr. Gustavo Rodriguez, an associate research scientist in the lab and first author on the manuscript, led the study. Geoffrey Barrett, second author on the paper, developed key applications for electrophysiology data analysis. Recently published in PLOS Biology, their manuscript highlights the important role neuronal hyperactivity plays in the progression of AD pathology.
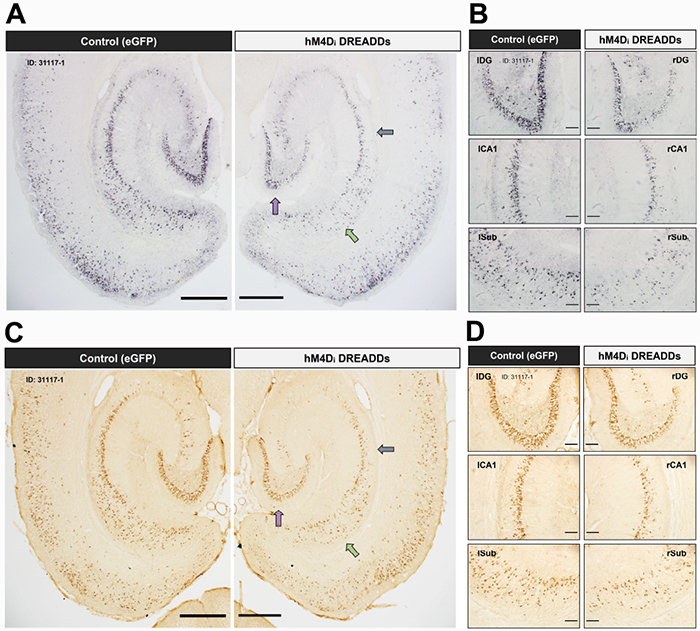
Figure 1: Chronic attenuation of neuronal activity in the entorhinal cortex reduces tau pathology in downstream hippocampal subregions. Low- and high-magnification images of immunostained brain sections are shown from a ~16-month EC-Tau/hAPP DREADD-activated mouse. Hyperphosphorylated tau (AT8+) (A-B) and abnormally conformed tau (MC1+) (C-D) are reduced in right versus left hemisphere. Figure adapted from Rodriguez et al (2020) PLOS Biology.
In this study, the authors use a newly developed mouse line (EC-Tau/hAPP) that exhibits age-dependent Aβ plaque accumulation in the brain, as well as intracellular tau aggregation in neurons along the EC-HIPP circuit. They first show that Aβ accumulation was associated with increased progression of tau pathology along the EC-HIPP circuit. Rodriguez et al. hypothesized that this was due to Aβ-associated disturbances in neuronal firing stemming from the EC, and sought to investigate this further using in vivo electrophysiology. Extracellular recordings were performed in the EC of 16-month EC-Tau/hAPP mice and littermate controls as they explored open field arenas. They found distinct signatures of neuronal hyperactivity and network dysfunction associated with Aβ accumulation, but not tau. They then used a chemogenetic approach (e.g. DREADDs, designer receptor exclusively activated by a designer drug) to combat the hyperactivity in the EC-Tau/hAPP model, with the goal of reducing the accumulation of Aβ and tau pathology along the EC-HIPP circuit. Six weeks of DREADDs activation reduced EC neuronal firing and network activity, and was effective at reducing Aβ accumulation, along with abnormally conformed and hyperphosphorylated tau aggregates, in the downstream hippocampus.
These data support the emerging view that Aβ-associated neuronal hyperactivity plays a role in Alzheimerâs disease pathogenesis, specifically by acting as an accelerant of tau spread along synaptically connected neuronal circuits in the brain.
Gustavo A. Rodriguez, PhD
Associate Research Scientist in the Taub Institute
gr2501@cumc.columbia.edu
Abid Hussaini, PhD
Assistant Professor of Pathology and Cell Biology (in the Taub Institute for Research on Alzheimer's Disease and the Aging Brain) at the Columbia University Medical Center
sah2149@cumc.columbia.edu
Alzheimer-Related Cerebrovascular Disease in Down Syndrome
 |  | |
| Patrick J. Lao, PhD | Adam M. Brickman, PhD |
There is much debate about the role of vascular risk factors and cerebrovascular dysfunction in Alzheimerâs disease. Most adults with Down Syndrome (DS) develop neuropathology and symptoms of Alzheimerâs disease (AD) early in life (age 40-60), but have low prevalence of traditional vascular risk factors. Magnetic resonance imaging (MRI) studies of adults with DS therefore provide a unique opportunity to both characterize cerebrovascular biomarkers in vivo and study cerebrovascular disease in AD without the confound of vascular risk factors. A new study from the laboratory of Dr. Adam Brickman, including associate research scientist and first author Patrick Lao, examined in vivo MRIâbased biomarkers of cerebrovascular pathology and their crossâsectional relationship with age, betaâamyloid pathology, and mild cognitive impairment or clinical AD diagnostic status, in a cohort of adults from the Biomarkers of Alzheimerâs Disease in Down Syndrome study (Dr. Nicole Schupf, Columbia PI).
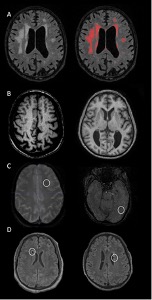
Figure 1: Examples of the cerebrovascular markers considered in the current study from typical study participants. (A) Distributed white matter hyperintensities displayed on T2âweighted fluidâattenuated inversion recovery (FLAIR) scan (left) labeled with inâhouseâdeveloped software (right). (B) Widespread enlarged perivascular spaces throughout the white matter appreciated on T1âweighted scans in 2 participants displayed in axial (left) and coronal (right) orientations. (C) Lobar microbleeds in 2 study participants displayed on axial susceptibilityâweighted images. (D) Cerebral infarcts in 2 participants displayed on axial T2âweighted FLAIR scans.
As recently published in the Annals of Neurology, they found detectable white matter hyperintensities (WMH), enlarged perivascular spaces, infarcts, and microbleeds (Figure 1) across the AD continuum in older adults with DS. Of particular interest, Lao et al. noted a monotonic increase in WMH volume, enlarged PVS, and presence of infarcts across diagnostic groups (cognitively stable<mild cognitive impairment<possible AD dementia<definite AD dementia). Their findings add to a growing body of evidence that implicates cerebrovascular disease as a core feature of AD and not simply a comorbidity.
Patrick J. Lao, PhD
Associate Research Scientist in the Gertrude H. Sergievsky Center
pjl2133@cumc.columbia.edu
Adam Brickman, PhD
Professor of Neuropsychology (in Neurology, the Taub Institute for Research on Alzheimer's Disease and the Aging Brain and the Gertrude H. Sergievsky Center)
amb2139@cumc.columbia.edu
 |  | |
| Rakshathi Basavaraju, MD | Frank Provenzano, PhD |
Frontotemporal dementia (FTD) is a devastating illness that is uniquely characterized by profound cortical loss and a wide range of behavioral phenomena, frequently overlapping with psychiatric conditions. Apathy and depression are among the most common of these conditions, though not always co-occurring. In the present study, recently published in the Journal of Geriatric Psychiatry and Neurology, Dr. Frank Provenzano and colleagues, including Dr. Edward Huey and first author Dr. Rakshathi Basavaraju (a post-doctoral research scientist in Dr. Provenzano's lab), sought to explore the differences between these two features using an open neuroimaging data set of FTD.
A challenge for open data neuroimaging is addressing uniform processing streams from site to site, as well as uniform quantitative pipelines. Developing a neuroimaging cortical thickness pipeline to account for statistical variance on this data, which varied greatly in terms of quality, permitted a larger and more heterogeneous group of FTD imaging. It is onto this inclusive dataset which the investigators sought to explore the relationship between apathy and depression.
 Figure 1: Anterior, Right lateral and Inferior surfaces of a 3D brain showing the areas (in blue) that are significantly of greater thickness in depressed versus patients who are not depressed in FTD (p‹0.05), R=Right
|
While evaluating apathy on these images, they discovered differences in several regions in the right side of the brain, including the middle and inferior frontal, superior and transverse temporal, and superior parietal and supramarginal gyri, as well as posterior cingulate and insula. Interestingly, depression was associated with thicker right-sided inferior and medial frontal cortex. This suggests a potential requisite threshold of the gray matter required for manifestation of depression in this population. These neuroimaging frameworks can be applied to other diseases with corresponding or similar behavioral measures and help add to the constellation of neuroimaging signatures of behavior, especially across multiple datasets and diagnoses.
Rakshathi Basavaraju, MD
Postdoctoral Research Scientist in the Taub Institute
rb3419@cumc.columbia.edu
Frank Provenzano, PhD
Assistant Professor of Neurological Sciences (in Neurology and the Taub Institute)
fap2005@cumc.columbia.edu

