Columbia University
Irving Medical Center
Neurological Institute
710 West 168th Street, 3rd floor
(212) 305-1818
TaubCONNECT Research Perspectives:
November 2018
2: » An MRI Measure of Degenerative and Cerebrovascular Pathology in Alzheimer Disease
3: » Integrative Transcriptome Analyses of the Aging Brain Implicate Altered Splicing in Alzheimer's Disease Susceptibility
» #1 A Multi-Omic Atlas of the Human Frontal Cortex for Aging and Alzheimer's Disease Research
» #2 Whole-exome Sequencing in 20,197 Persons for Rare Variants in Alzheimer's Disease
» #2 Preparation of Tau Oligomers After the Protein Extraction from Bacteria and Brain Cortices
» #2 Medical Retirement from Sport after Concussions: A Practical Guide for a Difficult Discussion
» #1 Cross Domain Self-Monitoring in Anosognosia for Memory Loss in Alzheimer's Disease
» #2 White Matter Changes in Alzheimer's Disease: A Focus on Myelin and Oligodendrocytes
» #1 ZCCHC17 is a Master Regulator of Synaptic Gene Expression in Alzheimer's Disease
» #2 Imaging Translocator Protein as a Biomarker of Neuroinflammation in Dementia
» #3 A Transcriptomic Atlas of Aged Human Microglia
» #1 Neuronal Lysosomal Dysfunction Releases Exosomes Harboring APP C-terminal Fragments and Unique Lipid Signatures
» #1 Neuronal Hyperactivity Due to Loss of Inhibitory Tone in APOE4 Mice Lacking Alzheimer's Disease-Like Pathology
and
» The Endosomal–Lysosomal Pathway Is Dysregulated by APOE4 Expression in Vivo
» First Place: A CSF Proteomic Screen Links Retromer to Alzheimer's Pathogenic Pathways and Suggests Endosomal-Trafficking Biomarkers
» First Place: Microglia Identity in the Aged and AD Human Brain
» #1 Intra-Axonal Synthesis of SNAP25 is Required for the Formation of Presynaptic Terminals
» #1 Stabilization of Dynamic Microtubules by mDia1 Drives Tau-dependent Aβ1-42 Synaptotoxicity
» #2 LTP and Memory Impairment Caused by Extracellular Aβ and Tau Oligomers is APP-Dependent
» #2 Neuropathologic Features of TOMM40 '523 Variant on Late-Life Cognitive Decline
» #2 An Approach to Studying the Neural Correlates of Reserve
» #1 Brain Atrophy Can Introduce Age-Related Differences in BOLD Response
» #2 Age-Related Biomarkers in LLFS Families With Exceptional Cognitive Abilities
» #2 Polygenic Risk Scores in Familial Alzheimer Diseases
» #1 Local Synthesis of Dynein Cofactors Matches Retrograde Transport to Acutely Changing Demands
» #3 Relation of Dysglycemia to Structural Brain Changes in a Multiethnic Elderly Cohort

Inbal Israely, PhD
The question of how neurons store information, while retaining the capacity to adapt to the environment, remains a critical issue in neuroscience. At the synaptic level, learning is thought to occur through activity driven weight changes termed Hebbian plasticity, which modify the firing rate of the cell. In addition, homeostatic plasticity (HSP) is a mechanism which keeps activity within a dynamic range, thus maintaining stability. HSP ensures that too much excitability does not build up (as may occur in epilepsy) or that neural networks become too quiet (as in Alzheimer’s Disease). However, it is unknown how functional and structural changes in neurons, and individual synapses in particular, are implemented when diverse forms of activity coincide. A new study by Taub investigator Dr. Inbal Israely and colleagues investigates the structural outcomes of homeostatic plasticity and the consequences of this for Hebbian plasticity when these forms of plasticity overlap at single spines.
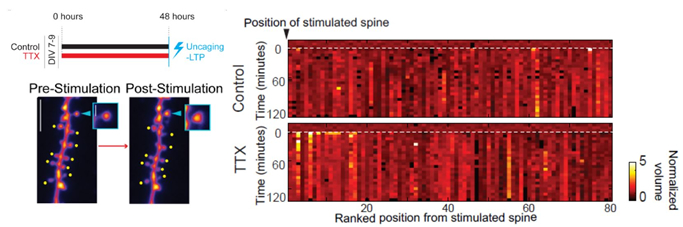 Figure: After homeostatic plasticity (induced with TTX), stimulation of a single spine with glutamate uncaging leads to changes also at neighboring inputs.
|
In their recently published study in iScience, Hobbiss et al. used 2-photon imaging in combination with glutamate uncaging to study the structural consequences of chronic activity blockade on hippocampal inputs, and how they subsequently respond to activity. They found that chronic activity blockade led to the enlargement of spines, resulting in structural scaling of inputs which retained their relative sizes. However, not all changes affected spines equally: large spines remained stable and did not grow further, while small inputs showed a reduced threshold for Hebbian plasticity - they grew robustly even in response to weak stimulation. While such activity normally changes only the stimulated spine, potentiation of a single synapse also led to growth of nearby inputs. “The fact that neighboring spines grew together with an active spine signifies that homeostatic plasticity changes one of the hallmark features of information storage, which is that plasticity is limited to the site of information entry," explains Dr. Israely. Thus, the different plasticity mechanisms which are at work in the neuron can cooperate to change which and how many inputs respond to a stimulus, and in so doing, may coordinate the physical connectivity of inputs in response to global changes in activity.
Inbal Israely, PhD
Assistant Professor of Pathology and Cell Biology (in Neuroscience and the Taub Institute)
ii2176@cumc.columbia.edu
An MRI Measure of Degenerative and Cerebrovascular Pathology in Alzheimer Disease
 |  |  | ||
| Adam M. Brickman, PhD | Giuseppe Tosto, MD, PhD | Richard Mayeux, MD, MSc |
Although the contribution of cerebrovascular disease (CVD) to late-onset Alzheimer's disease (LOAD) continues to be debated, CVD is present in up to 70% of patients compared with only 15%–20% of healthy elderly at postmortem examination and mixed pathology is now considered a primary cause of Alzheimer-related dementia. On magnetic resonance imaging (MRI), LOAD is manifest by medial temporal lobe and cortical atrophy, but white matter hyperintensities (WMHs)—presumed to be an indication of small vessel cerebrovascular pathology—and brain infarcts are also frequent. Structural MRI biomarker studies of LOAD often treat CVD and atrophy as distinct entities, but they coexist more frequently than not and the onset of clinical symptoms in LOAD may be jointly determined by both entities.
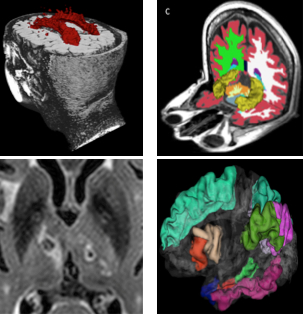
Figure: The four components that comprised the MRI summary score included white matter hyperintensities (top left), infarcts (bottom left), hippocampal volume (top right), and cortical thickness (bottom right).
In a new study, published recently in Neurology, with an accompanying editorial, Drs. Adam Brickman, Guiseppe Tosto, Richard Mayeux, and colleagues from Taub sought to develop a quantitative MRI measure reflecting the joint contributions of WMH, brain infarcts, and neurodegeneration to the clinical and pathologic diagnosis of LOAD. The quantitative MRI measure was derived in the community-based Washington Heights-Inwood Columbia Aging Project cohort by linearly combining individual cerebrovascular and neurodegenerative factors weighted by their association with episodic memory. Brickman et al. forward applied the score to independent samples and performed further validation by demonstrating its association with neuropathology at autopsy, LOAD biomarkers, and conversion from mild cognitive impairment to LOAD. These results confirm and augment the role of both neurodegeneration and cerebrovascular pathology in LOAD, and suggest the possibility of capturing both in a single valid and reliable MRI-based measure.
Adam M. Brickman, PhD
Associate Professor of Neuropsychology (in Neurology, the Gertrude H. Sergievsky Center and the Taub Institute)
amb2139@cumc.columbia.edu
Giuseppe Tosto, MD, PhD
Assistant Professor of Neurology (in the Gertrude H. Sergievskt Center and the Taub Institute)
gt2260@cumc.columbia.edu
Richard Mayeux, MD, MSc
Gertrude H. Sergievsky Professor of Neurology, Psychiatry and Epidemiology (in the Gertrude H. Sergievsky Center and in the Taub Institute)
rpm2@cumc.columbia.edu

Philip L. De Jager, MD, PhD, MMSc
Alternative splicing is an important posttranscriptional regulatory mechanism through which pre-mRNA molecules can produce multiple distinct mRNAs. Disruptions in RNA metabolism, including mRNA splicing, are associated with age-related disorders, such as frontotemporal lobar dementia, Parkinson's disease, and Alzheimer's disease (AD). A new study by Taub investigator Dr. Philip L. De Jager, Dr. Towfique Raj (Mount Sinai), and colleagues used deep sequencing to identify sources of variation in mRNA splicing in a large dataset of aging brains. By applying state-of-the-art analytic methods, they were able to generate a comprehensive genome-wide map of splicing variation in the aging prefrontal cortex.
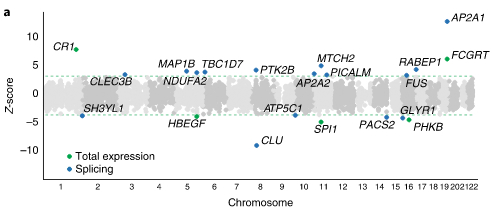
As published recently in Nature Genetics, Raj et al. report that altered splicing is the mechanism for the effects of the PICALM, CLU and PTK2B susceptibility alleles. Further, they identified 21 genes with significant associations with Alzheimer's disease, many of which are found in known loci, whereas eight are in novel loci. "This study of the transcriptome of the aging brain provides evidence that dysregulation of mRNA splicing is a feature of Alzheimer's disease and is, in some cases, genetically driven," concludes Dr. De Jager and colleagues. "The catalog of splicing variants made available with this study provides a starting point for further focused molecular and biochemical experimental validation to fully elucidate the role of these splicing variants in the etiology of Alzheimer's disease."
|
Figure 5a: New genes discovered by the transcriptome-wide (green) and spliceosome wide (blue) association studies.
|
Philip L. De Jager, MD, PhD, MMSc
Weil-Granat Professor of Neurology (in the Taub Institute, the Precision Medicine Initiative, and the Center for Translational and Computational Neuro-immunology)
pld2115@cumc.columbia.edu