Columbia University
Irving Medical Center
Neurological Institute
710 West 168th Street, 3rd floor
(212) 305-1818
TaubCONNECT Research Perspectives:
March 2018
2: » White Matter Changes in Alzheimer's Disease: A Focus on Myelin and Oligodendrocytes
Cross Domain Self-Monitoring in Anosognosia for Memory Loss in Alzheimer's Disease
 |  |
|
| Silvia Chapman, MS | Stephanie Cosentino, PhD |
Anosognosia, or unawareness of memory loss, is a common feature of Alzheimer's disease (AD). This disorder in self-awareness has been linked to a variety of negative personal and societal consequences. There is a growing yet incomplete understanding of the ways in which self-awareness breaks down in AD. Existing models of anosognosia, or global awareness, have outlined the ways in which dysfunctional cognition, particularly memory and executive functioning, may produce disordered self-awareness. However, as disruptions to memory and executive function do not fully explain anosognosia in AD, it is clear that other mechanisms are at play in the deterioration of self-awareness. Currently, there is a drive in both the cognitive and motor literatures towards a dynamic and multifaceted notion of self-awareness wherein factors specific to metacognition, or knowledge of one’s own cognition, are key for producing this fascinating disorder.
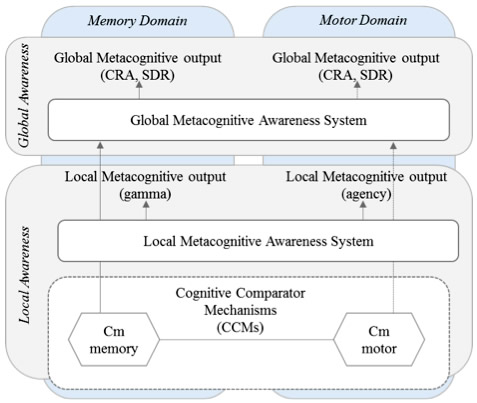
Figure: Solid lines represent relationships found in this current study. Dotted arrows represent relationships previously shown in anosognosia for hemiplegia but not assessed in this study (see Jenkinson & Fotopoulou, 2010; Saj et al., 2014; Vocat et al., 2013). CRA – Clinically rated awareness; SRD – Subjective rating discrepancy; Cm – memory comparator; Cn – motor comparator (Agnew & Morris, 1998).
In this vein, the Taub Institute laboratory of Dr. Stephanie Cosentino has developed a novel line of research dedicated to examining how disruption to different types of metacognition, or self-evaluative processes, may contribute to anosognosia in AD. Previous work from the Cosentino lab showed that individuals with anosognosia have difficulty with self-awareness at a more "local" level, that is, with monitoring their memory from moment to moment. In the present study, recently published in Cortex, Dr. Cosentino, lead author Silvia Chapman, and colleagues asked whether disruption to this local level of self-monitoring is domain specific or whether it is present across different functional domains. Both memory and motor monitoring were examined in relation to anosognosia in a group of 35 patients with AD recruited from the Department of Neurology Division of Aging and Dementia. Interestingly, while results supported a "cross-domain" association between memory and motor monitoring processes, only memory monitoring was associated with anosognosia. That is, deterioration of awareness in AD was found to occur in a domain specific manner. Results have implications for current models of awareness (see figure 1), such that two separate levels of awareness seem to exist, and lower levels feed higher levels within a specific functional domain.
Silvia Chapman, MS
Staff Associate in the Taub Institute
sc4056@cumc.columbia.edu
Stephanie Cosentino, PhD
Associate Professor of Neuropsychology (in Neurology, the Gertrude H. Sergievsky Center and the Taub Institute for Research on Alzheimer's Disease and the Aging Brain)
sc2460@cumc.columbia.edu
White Matter Changes in Alzheimer's Disease: A Focus on Myelin and Oligodendrocytes
 |  | |
| Sara Ebrahimi Nasrabady, MD/PhD | Adam M. Brickman, PhD |
The Taub Institute laboratory of Dr. Adam Brickman uses neuroimaging technologies to elucidate brain-behavior relationships related to cognitive aging and neurodegenerative disorders. A series of recent observations showed that white matter abnormalities visualized on MRI scanning are related to risk, course, and genetics of Alzheimer’s disease (AD) and likely represent a core feature of the disorder. Using translational neuroscience approaches, the laboratory is turning to the histopathology of brain tissue to elucidate the mechanistic underpinnings of these radiological abnormalities.
A recent review article in Acta Neuropathologica Communications led by Dr. Sara Ebrahimi Nasrabady, a postdoctoral research fellow in Dr. Brickman's laboratory, together with CUIMC collaborator Dr. James E. Goldman and graduate student Batool Rizvi, probed a variety of structural, histopathological, and biochemical pathologies that characterize the white matter of AD patients. Their review describes evidence for white matter abnormalities in AD, with a focus on myelin and oligodendrocytes, and discusses the relationship between these white matter changes and the hallmark pathological features of AD.
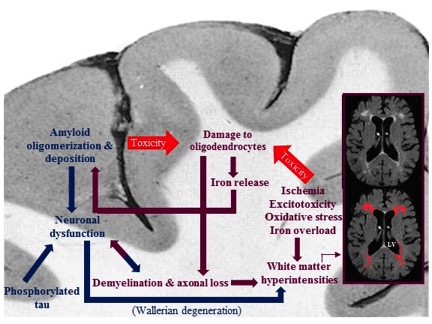
Figure: Summary of the pathological cascades, and their relation with each other, occurring during the development of Alzheimer's disease in white matter and cortex. While ischemia, excitotoxicity, oxidative stress, and iron overload in white matter damage oligodendrocytes, on one hand, and amyloid toxicity affects them, on the other hand, the iron released from damaged oligodendrocytes promotes amyloid polymerization and deposition in grey matter. The consequent demyelination and axonal loss result in further white matter damage and neuronal dysfunction. Neuronal dysfunction is also a result of amyloid deposition in cortex and a proposed cause for white matter abnormalities in AD patients.
In AD, oligodendrocytes, or their precursors, responsible for remyelination of damaged white matter areas are altered in number and in DNA stability, and are functionally less efficient in the presence of genetic changes, excitotoxicity, oxidative stress, increased iron levels, hypoxia/ischemia and vascular pathology that are common in AD. Here, Nasrabady et al. conclude that white matter abnormalities—impaired myelin and oligodendrocytes, in particular—promote cognitive impairment and AD pathology, and may be important preventative or treatment targets.
Sara Ebrahimi Nasrabady, MD/PhD
Postdoctoral Research Fellow in the Late-life Neuropsychiatric Disorders Research Training program (T32), Department of Psychiatry
se2351@cumc.columbia.edu
Adam M. Brickman, PhD
Associate Professor of Neuropsychology in Neurology, the Taub Institute for Research on Alzheimer's Disease and the Aging Brain and the Gertrude H. Sergievsky Center
amb2139@cumc.columbia.edu

