Columbia University
Irving Medical Center
Neurological Institute
710 West 168th Street, 3rd floor
(212) 305-1818
TaubCONNECT Research Perspectives:
December 2018
2: » Semantic Network Function Captured by Word Frequency in Nondemented APOE ε4 Carriers
 |
 |
|
| Hans-Ulrich Klein, PhD | Philip L. De Jager, MD, PhD |
Epigenetic mechanisms are involved in aging and the pathogenesis of neurodegenerative diseases like Alzheimer’s disease (AD). Alterations of the epigenome can mediate the effect of genetic or environmental risk factors, or occur as a consequence of the disease and mediate the toxicity of pathologies aggregating in the AD brain. Notably, tau pathology has recently been associated with epigenomic changes in model systems. In Drosophila, tau overexpression has been shown to relax heterochromatin. There is also evidence for a physiological function of nuclear tau in maintaining and regulating heterochromatin, which may be lost by pathologically phosphorylated tau. However, whether these mechanisms cause major chromatin alterations in the human AD brain, translate to transcriptional alterations, and are restricted to heterochromatic regions remains unknown.
As recently reported in Nature Neuroscience, Taub investigators Drs. Hans-Ulrich Klein and Philip L. De Jager studied the acetylation of the ninth lysine of histone 3, which marks transcriptionally active open chromatin genome-wide in the dorsolateral prefrontal cortex of 669 subjects. Their findings support the hypothesis that tau but not amyloid-β causes widespread chromatin remodeling. Tau- related alterations aggregated in large genomic segments reflecting spatial chromatin organization, and the magnitude of these effects correlated with the segment’s nuclear lamina association (see Figure). The effect of these epigenomic changes propagated into the transcriptome and could be replicated in tau mouse models. Further, the authors employed iPSC-derived neurons to demonstrate that tau is sufficient to induce major chromatin remodeling and that an Hsp90 inhibitor (17-DMAG), that is computationally predicted to block the effects of tau based on their human brain data, could attenuate the chromatin alterations induced by tau.
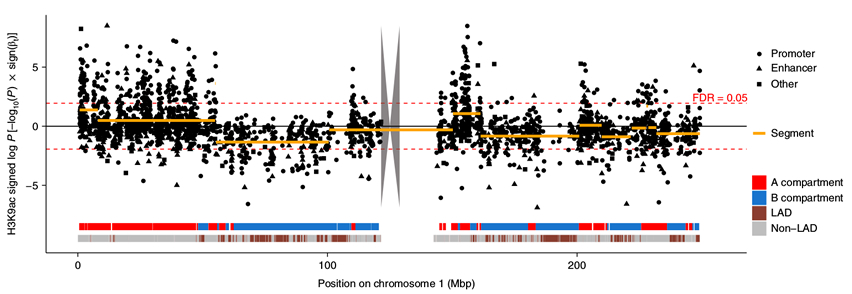 Figure: Tau-related chromatin alterations show spatial patterns. Manhattan plot depicts log-transformed P values for the tau effect for all H3K9ac domains on chromosome 1. The signs of the transformed P values reflect the directionality of the effects. Broad genomic segments covering H3K9ac domains showing associations with tau similar in direction and strength are depicted as orange lines. Ribbons at the bottom show chromatin-structure-related annotation.
|
Hans-Ulrich Klein, PhD
Assistant Professor of Neurological Sciences (in Neurology, the Taub Institute for Research on Alzheimer's Disease and the Aging Brain)
Columbia University Irving Medical Center
hk2948@cumc.columbia.edu
Philip L. De Jager, MD, PhD
Weil-Granat Professor of Neurology (in Neurology, the Taub Institute for Research on Alzheimer's Disease and the Aging Brain and the Precision Medicine Initiative)
pld2115@cumc.columbia.edu
 |  | |
| Jet M. J. Vonk, PhD | Jennifer J. Manly, PhD |
Semantic Network Function Captured by Word Frequency in Nondemented APOE ε4 Carriers
A convincing body of evidence from longitudinal studies has shown that cognitive change within individuals affected by Alzheimer's disease (AD) occurs years, even decades before clinical threshold for dementia occurs. Accurate identification of early cognitive changes associated with AD is critically needed to support early detection. While biological markers are beginning to dominate research on the long "preclinical" phase of Alzheimer's disease, it is possible that sensitive predictors of early cognitive change may be hidden within affordable and accessible existing neuropsychological tests. Semantic fluency—generating as many exemplars of a category within one minute—is typically used in dementia evaluations because it is sensitive to mild cognitive impairment (MCI) and dementia, but has not been proven useful in the preclinical phase. When examined at the item level, however, fluency measures harbor valuable information about the integrity of semantic networks affected early in AD.
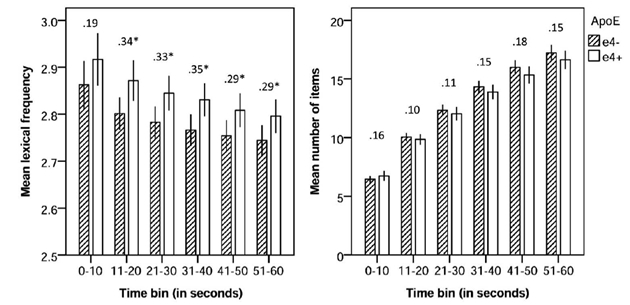 Figure 1: Animal fluency performance across task time (number above bars represent effect size in Cohen’s d; 95% confidence interval error bars). * p = .05.
|
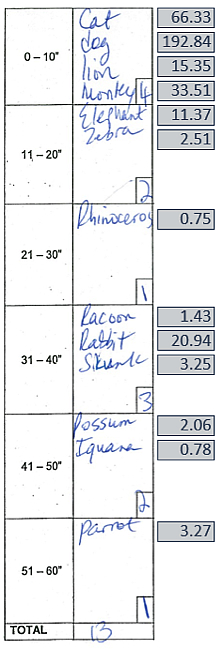
Figure 2: Semantic fluency task with total number score and lexical frequency label per 1 million words (via SUBTLEXus; e.g., dog occurs ±193 times per million words)
Trained as a neurolinguist, postdoctoral fellow and first author Dr. Jet Vonk identified a unique psycholinguistic measure based on the lexical frequency—how often a word is used in our language. Together with Taub Institute investigators Drs. Jennifer Manly and Adam Brickman, Dr. Vonk examined whether lexical frequency differentiated APOE ε4 carriers from noncarriers among 230 older cognitively healthy African American adults without dementia or MCI. As published in Neuropsychology, their study demonstrates that APOE ε4 carriers generate more frequent words (e.g., dog, horse, bird) than noncarriers, who were more likely to generate low frequency words (e.g., lynx, platypus, puma). Lexical frequency was particularly sensitive to ε4 status after the first 10 seconds of the test. In contrast, no group differences were found in the traditional way of scoring fluency measures, total number of words generated. These findings led Vonk et al. to conclude that "psycholinguistic analyses of fluency data have the potential to increase utility of neuropsychological instruments in detection of Alzheimer’s disease risk within the preclinical stage and thus may refine the definition of high-risk populations for clinical trials."
Jet M. J. Vonk, PhD
Postdoctoral Research Scientist (in the Gertrude H. Sergievsky Center)
jv2528@cumc.columbia.edu
Jennifer J. Manly, PhD
Professor of Neuropsychology (in Neurology, the Gertrude H. Sergievsky Center and the Taub Institute)
jjm71@cumc.columbia.edu

