Columbia University
Irving Medical Center
Neurological Institute
710 West 168th Street, 3rd floor
(212) 305-1818
TaubCONNECT Research Perspective:
October 2022
2: Diet Moderates the Effect of Resting State Functional Connectivity on Cognitive Function
3: Longitudinal Patterns of Cortical Atrophy on MRI in Patients With Alzheimer Disease With and Without Lewy Body Pathology
Retromer Deficiency in Tauopathy Models Enhances the Truncation and Toxicity of Tau
Progranulin Mutations in Clinical and Neuropathological Alzheimer's Disease
Wolframin is a Novel Regulator of Tau Pathology and Neurodegeneration
Homotypic Fibrillization of TMEM106B Across Diverse Neurodegenerative Diseases
Correlation of Plasma and Neuroimaging Biomarkers in Alzheimer's Disease
Tubulin Tyrosination Regulates Synaptic Function and is Disrupted in Alzheimer's Disease
The Penalty of Stress - Epichaperomes Negatively Reshaping the Brain in Neurodegenerative Disorders
The Neuronal Retromer can Regulate Both Neuronal and Microglial Phenotypes of Alzheimer's Disease
Deep Learning Improves Utility of Tau PET in the Study of Alzheimer's Disease
Age of Onset of Huntington's Disease in Carriers of Reduced Penetrance Alleles
Caspase-9: A Multimodal Therapeutic Target With Diverse Cellular Expression in Human Disease
Midlife Vascular Factors and Prevalence of Mild Cognitive Impairment in Late-Life in Mexico
The Association Between Sex and Risk of Alzheimer's Disease in Adults with Down Syndrome
Marked Mild Cognitive Deficits in Humanized Mouse Model of Alzheimer's-Type Tau Pathology
Rapid ATF4 Depletion Resets Synaptic Responsiveness after cLTP
Polygenic Risk Score for Alzheimer's Disease in Caribbean Hispanics
Recognition Memory and Divergent Cognitive Profiles in Prodromal Genetic Frontotemporal Dementia
The Microtubule Cytoskeleton at the Synapse & The Synaptic Life of Microtubules
Optimizing Subjective Cognitive Decline to Detect Early Cognitive Dysfunction
The AD Tau Core Spontaneously Self-Assembles and Recruits Full-Length Tau to Filaments
Olfactory Impairment is Related to Tau Pathology and Neuroinflammation in Alzheimer's Disease
Pathogenic Role of Delta 2 Tubulin in Bortezomib-Induced Peripheral Neuropathy
Clearance of an Amyloid-Like Translational Repressor is Governed by 14-3-3 Proteins
 |  | |
| Grace Herod, PhD | Luke E. Berchowitz, PhD |
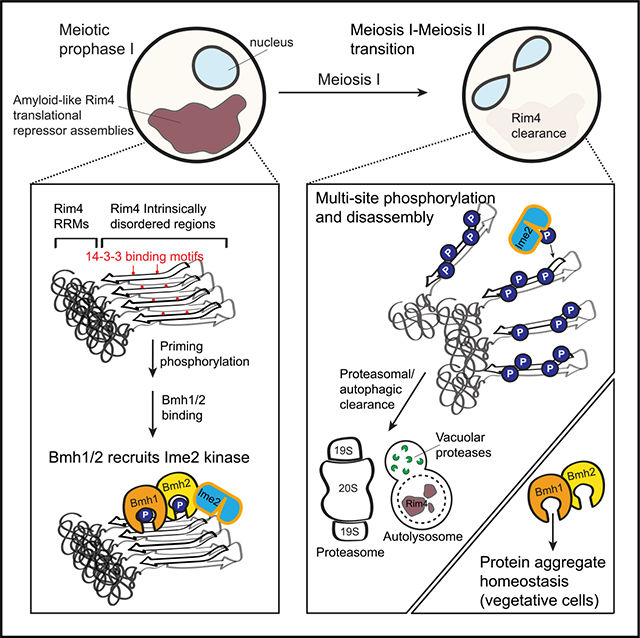
Amyloids arise from the repetitive addition of multiple copies of a protein with consequent conformational alterations. These fibrous assemblies are typically associated with the etiology of devastating age-related diseases such has Alzheimer’s disease and transthyretin amyloidosis. Recently, the paradigm that amyloids are intrinsically irreversible has been challenged by evidence indicating that some cells use reversible amyloid assemblies to carry out important functions. This means that healthy cells have evolved unknown mechanisms to dismantle these extremely stable structures which has vital implications for health and disease. In an effort led by graduate student Grace Herod (now Dr. Herod) in the Berchowitz lab, we sought to determine the mechanistic underpinning of cells’ remarkable capacity reverse and clear amyloids.
To address this question, we used a simple tractable model system: budding yeast gametes. During development, these cells use reversible amyloid-like assemblies of the RNA-binding protein Rim4 to repress translation of mRNAs that encode protein products that are harmful when prematurely expressed. Rim4 assemblies are termed ‘amyloid-like’ because they share a subset of the biochemical properties associated with disease-related amyloids such as resistance to ionic detergents and formation of stable cross-Β-sheet structures. Before this study, we knew that phosphorylation played a critical role in Rim4 clearance; a modification which also plays into the etiology of several disease-related amyloids. However, a mechanistic understanding of the contribution of phosphorylation to the clearance of amyloids and amyloid-like assemblies such as Rim4 clearance was lacking.
In a paper published this year (2022) in Cell Reports, Grace found that Rim4 amyloid-like assemblies physically interact with a class of proteins called 14-3-3 proteins. This was exciting because many neurodegenerative disease-associated aggregates are bound by 14-3-3 proteins. Furthermore, 14-3-3 often act via their ability to bind phosphorylation modifications. She went on to demonstrate that the interaction between Rim4 and 14-3-3 proteins is critical for timely disassembly and clearance of Rim4. Grace’s results support a model by which 14-3-3 proteins play non-redundant roles in facilitating critical priming phosphorylation events on amyloid-like Rim4. Remarkably, Grace was able to expand the breadth of her findings by discovering that 14-3-3 proteins contribute to global protein aggregate homeostasis. Together, this study provides mechanistic model describing how 14-3-3 proteins may interact with phosphorylated residues to regulate and protect against disease-associated protein aggregates.
Luke E. Berchowitz, PhD
Assistant Professor of Genetics and Development (in the Taub Institute for Research on Alzheimer's Disease and-the Aging Brain)
leb2210@cumc.columbia.edu
Diet Moderates the Effect of Resting State Functional Connectivity on Cognitive Function
 |  | |
| Alexandra M. Gaynor, PhD | Christian Habeck, PhD | |

|  | |
| Yaakov Stern, PhD | Yian Gu, MD, MS, PhD |
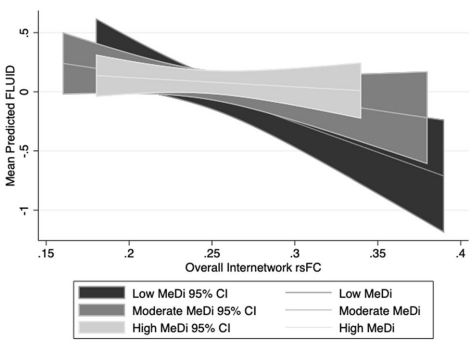
Adherence to a Mediterranean diet (MeDi) has been associated with lower incidence of Alzheimer’s Disease (AD), lower risk of mild cognitive impairment (MCI), and slower cognitive decline in multiple domains. Relatively little research has investigated the relationship between diet and brain imaging biomarkers, but a small number of studies, including previous research from our group, have shown that MeDi adherence is associated with structural measures of brain health such as brain volume, cortical thickness, and structural connectivity. However, no prior research had examined the effects of MeDi on brain function as measured by fMRI. Meanwhile, work from our group has demonstrated that variability in cognitive performance during aging may depend on organization of functional brain networks, and increased internetwork resting-state functional connectivity (rsFC), which reflects less differentiation between functional networks, is associated with aging and poorer cognitive performance. Given evidence that MeDi impacts structural brain health, and that other lifestyle factors have been shown to moderate associations between brain measures and performance, it is possible that MeDi also plays a role in the relationship between rsFC and cognitive function.
In a recent study, we tested whether MeDi moderates the associations between internetwork rsFC and cognitive performance. A sample of 201 cognitively intact adults 20–80 years old underwent resting state fMRI to measure rsFC among 10 networks, and completed 12 cognitive tasks assessing perceptual speed, fluid reasoning, episodic memory, and vocabulary. As published last month in Scientific Reports, we found that MeDi was not directly associated with rsFC, but significantly moderated the relationship between internetwork rsFC and fluid reasoning performance: in individuals with low adherence to MeDi, there was a significant negative association between overall internetwork rsFC and performance, but this effect was insignificant in those with moderate and high MeDi adherence.
Overall, our results suggest MeDi may support cognitive function by attenuating the negative effect of internetwork connectivity on cognitive function. Our findings could potentially be interpreted in the context of cognitive reserve (CR), the properties of the brain that allow for better than expected cognitive performance given the degree of injury- or age-related changes to the brain. Future research is warranted to understand how MeDi impacts structural and functional brain biomarkers and cognitive function over time, and to understand which precise patterns of nutrient intake may provide optimal protection against neurocognitive decline.
Alexandra M. Gaynor, PhD
Postdoctoral Research Scientist in the Taub Institute
ag4498@cumc.columbia.edu
Yian Gu, MD, MS, PhD
Assistant Professor of Neurological Sciences (in Neurology, Epidemiology in the Gertrude H. Sergievsky Center and the Taub Institute)
yg2121@cumc.columbia.edu
 |  | |
| Allison Constant, MD | Lawrence S. Honig, MD, PhD | |

|  | |
| Karen S. Marder, MD, MPH | Frank Provenzano, PhD |
Although Alzheimer’s Disease (AD) and Lewy Body Dementia (LBD) represent two different pathologies, they have substantial clinical overlap. In fact, of the spectrum of neurodegenerative disorders, AD and LBD are found to overlap most frequently, with up to 80-89% of patients diagnosed with LBD having some degree of AD pathology at autopsy. Evidence of the co-occurrence of these diseases can become especially difficult given that the have distinct neuropathological profile of beta-amyloid plaques, neurofibrillary tangles in AD and alpha-synuclein deposits in LBD. One common tool that has traditionally not shown promise in disambiguating LBD in the presence of AD is magnetic resonance imaging (MRI); however, previous studies have not capitalized on the longitudinal (changes of time) patterns of loss in AD and AD/LBD patients nor do they have confirmed neuropathology.
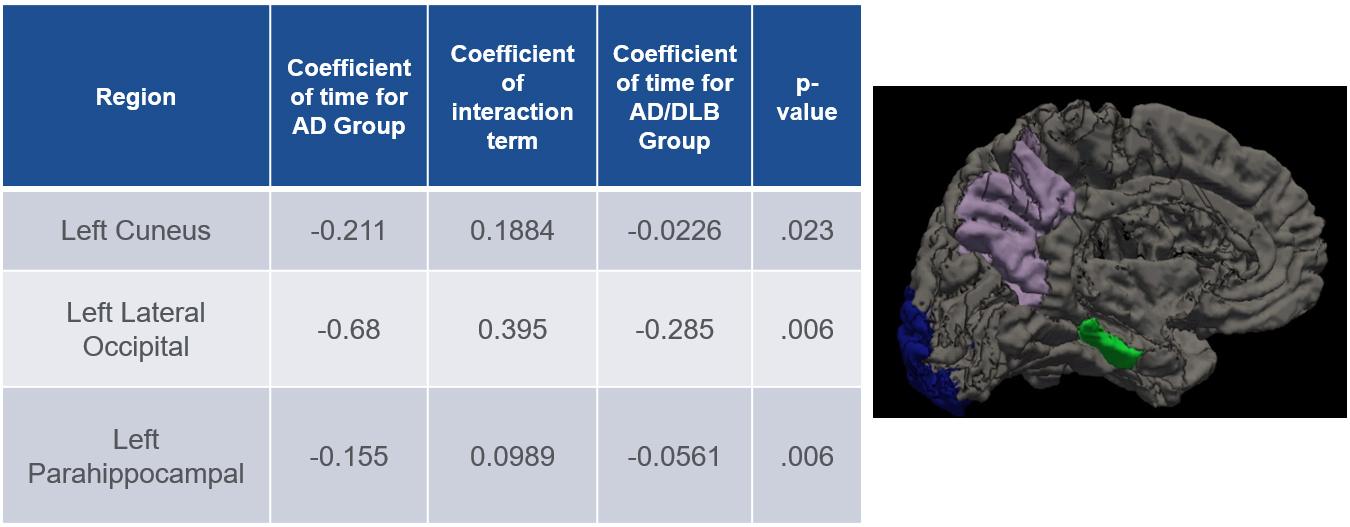 Figure 1. On the left table is the results of our linear mixed effects model analysis in AD vs LBD/AD showing atrophy over time for the three individual regions. On the right is a figure showing the three individual regions in an inflated left-brain hemisphere, Parahippocampal (green), cuneus (purple) and lateral occipital (blue).
|
In a study by the Provenzano lab recently published in Neurology, we sought to use a well-characterized longitudinal neuroimaging dataset of both AD and AD/LBD patients with confirmatory neuropathology to test if statistical models sensitive to longitudinal changes could distinguish LBD in patients with AD. Here, together with first author and Dean’s Research Fellow Dr. Allison Constant (P&S ‘22) and Drs. Karen Marder and Lawrence Honig, we sought to explore serial neuroimaging in 48 individuals with AD (n=24) and AD/LBD (n=24).
Anchoring this with neuropathological data from ADNI, we discovered that, when controlling for clinical disease severity among other factors, individuals with AD alone experienced higher rates of atrophy in the left cuneus, lateral occipital, and parahippocampal regions over time, when compared to patients with concomitant LBD using a linear mixed effect model analysis (p=0.22, 0.006 and 0.006 respectively). Furthermore, we found that the lattermost left cuneus volume positively correlated with Braak Lewy scores (Pearson product correlation 0.37, p=0.009) and left parahippocampal volume negatively correlated with Braak NFT score (-0.327, p=0.02), both neuropathological measures of disease burden. These findings suggest that longitudinal neuroimaging techniques may be useful in disambiguating disease progression trajectories in lieu of or in addition to other neuroimaging measures like PET scanning or dopamine sensitive SPECT scans.
Frank Provenzano, PhD
Assistant Professor of Neurological Sciences (in Neurology and in the Taub Institute for Research on Alzheimer's Disease and the Aging Brain)
fap2005@cumc.columbia.edu