Columbia University
Irving Medical Center
Neurological Institute
710 West 168th Street, 3rd floor
(212) 305-1818
TaubCONNECT Research Perspective:
June 2022
2: Single Cell/Nucleus Transcriptomics Comparison in Zebrafish and Humans Reveals Common and Distinct Molecular Responses to Alzheimer's Disease
 |
 |  | |
| Dallin Dressman, PhD Candidate | Badri N. Vardarajan, PhD, MS | Wassim Elyaman, PhD |
Alzheimer’s disease (AD) is a progressive and neurodegenerative disease of aging. The resident central nervous system's innate immune cells, microglia, have been the focus of these efforts thus far. However, there is also a vital need to understand the role of infiltrating adaptive immune cells, specifically T cells, that may either maintain healthy neurons in the affected brain regions or lead to neuronal loss in disease contexts.
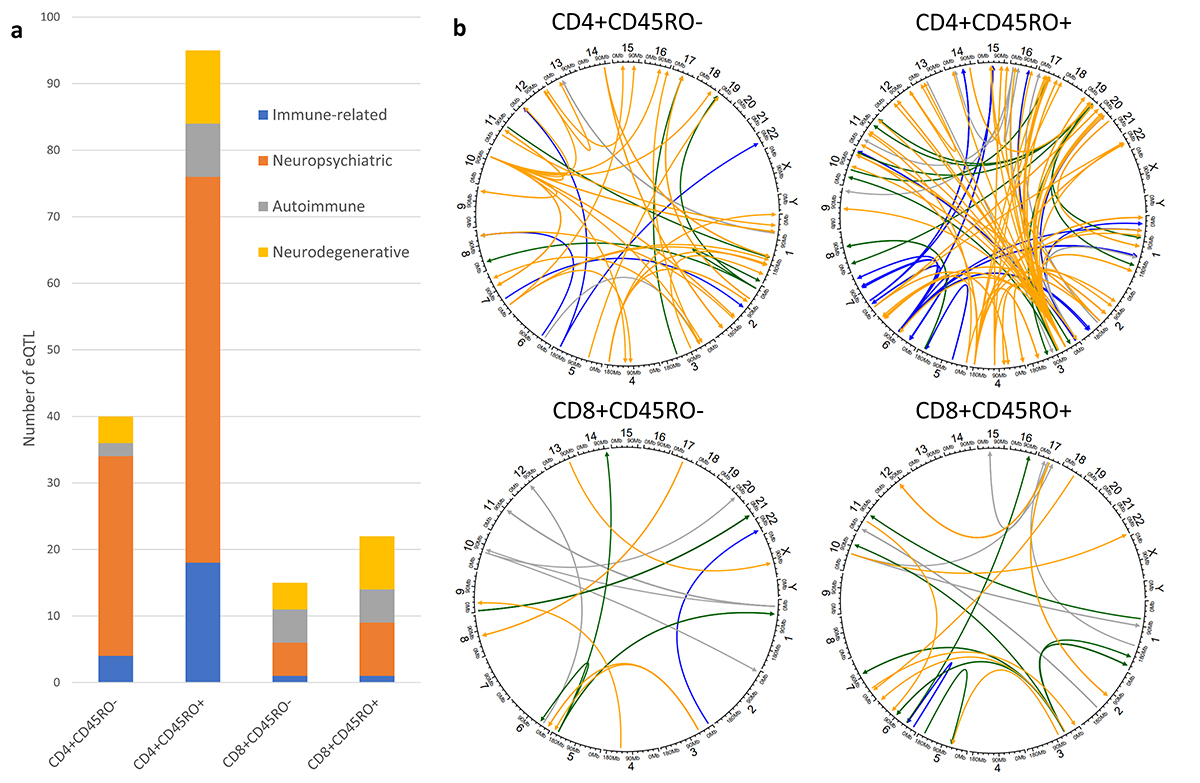
Figure 1. Disease association of trans-eQTL. a) Stacked bar graph showing numbers of trans-eQTL whose SNP is found to associate with certain categories of disease traits as found in the GWAS catalog. Several dozen SNPs among the trans-eQTLs have been shown to associate with traits related to immune function, neuropsychiatric conditions, autoimmune disease, or neurodegenerative disease, in genome-wide association studies. b) Circos plots displaying the distribution of disease-associated trans-eQTL by chromosome. Color legend for GWAS categories is found on the bar graph. Most of these SNPs were trans-eQTLs from CD4+CD45RO+ cells, with neuropsychiatric conditions featuring prominently among the disease-associated SNPs in this cell type. Interestingly, among the disease-associated eQTLs in CD4+CD45RO+ cells were several immunosenescence and cytotoxicity-related genes, including GNLY, GZMH, NKG7, and PRF1, all of which were regulated by SNPs associated with anxiety.
Recent studies identifying expression quantitative trait loci (eQTL) in immune cells have uncovered essential links between disease risk alleles and gene expression trends in monocytes, T cells, and other cell types. However, these studies are generally done with young, healthy subjects, limiting the utility of their findings for age-related conditions such as AD. In our publication in Human Molecular Genetics, we have performed RNA sequencing on four T cell subsets in genome-wide genotyped and well-characterized AD subjects and age- and sex-matched healthy controls from the Religious Orders Study/Memory and Aging Project at Rush University. Correlating gene expression data with AD neuropathological traits, and with single nucleotide polymorphisms to detect eQTLs, we identified several significant genes involved in T cell senescence and cytotoxicity, consistent with T cell RNA sequencing studies in aged/AD cohorts. We identified unexpected eQTLs previously associated with neuropsychiatric disease traits. Finally, we discovered that pathways related to axon guidance and synaptic function were enriched among trans-eQTLs in coding regions of the genome. Overall, our data sheds more light on the genetic basis behind phenotypic changes in T cells during aging and AD. Our study is the first, to our knowledge, to examine the genetic underpinnings of T cell expression phenotypes in aged individuals and AD patients.
Wassim Elyaman, PhD
Assistant Professor of Neurological Sciences (in Neurology, the Taub Institute and the Institute for Genomic Medicine)
we2152@cumc.columbia.edu
Badri N. Vardarajan, PhD, MS
Assistant Professor of Neurological Science (in Neurology, the Gertrude H. Sergievsky Center, and the Taub Institute)
bnv2103@cumc.columbia.edu
 |
 |  | |
| Mehmet Ilyas Cosacak, PhD | Giuseppe Tosto, MD, PhD | Caghan Kizil, PhD |
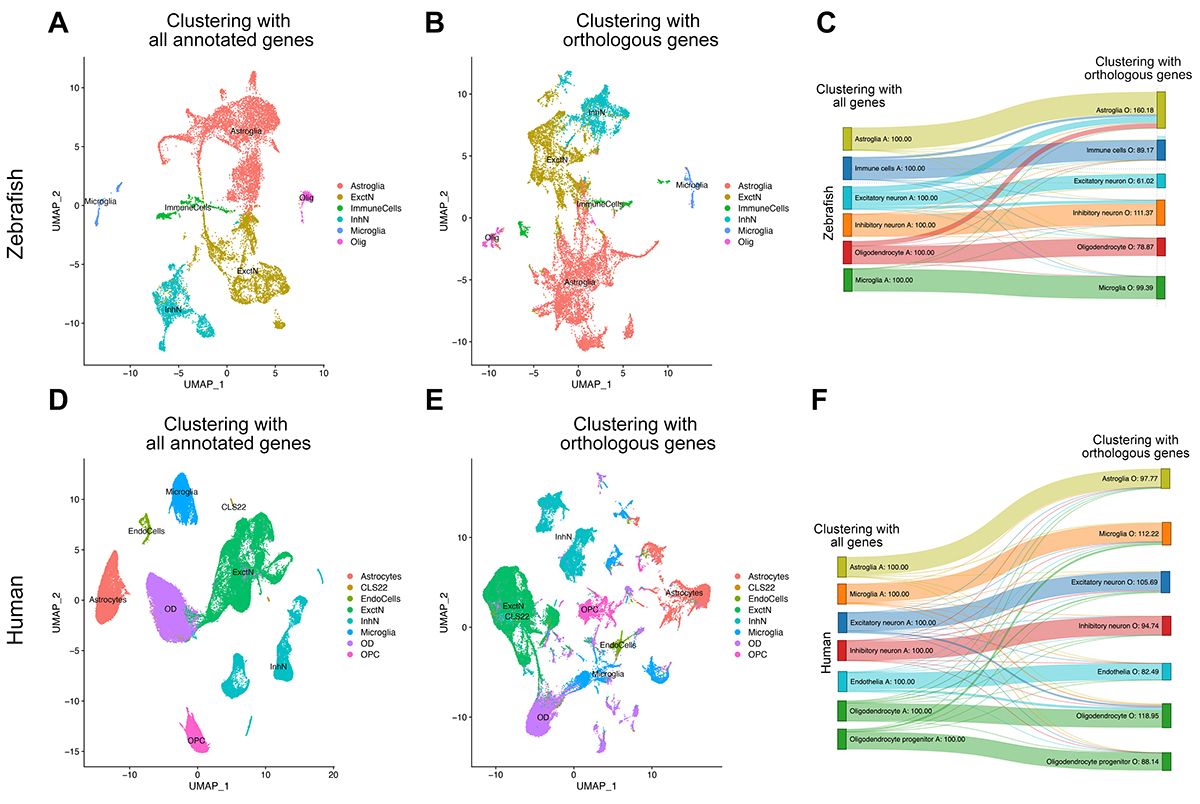
Understanding the biological mechanisms of genes or pathways related to human disease significantly benefits from animal models. Yet, these models are prone to limitations, such as potential differences in the physiology of the cells or tissues being investigated to human counterparts. Therefore, it is important to know which aspects of an animal model is relevant to human disease and suitable for comparative functional studies. In our recent publication in Cells, we compared the single cell transcriptomics from human brains with Alzheimer’s disease to adult zebrafish brain model of amyloid toxicity. Our comparison revealed that in evolutionarily divergent vertebrates - human and zebrafish— neurons respond to amyloid aggregation surprisingly similarly, while astroglial response had differences that are related to neurogenesis pathways.
In different neuronal subtypes, human and zebrafish responded remarkably similarly to amyloid toxicity. The molecular pathways affected in disease included clearance, energy metabolism and synaptic signalling, suggesting that addressing neuron-related pathological aspects of Alzheimer’s would be feasible in zebrafish. In microglial cells, we also determined common molecular reactions in humans and zebrafish. These included energy metabolism, antigen presentation and chemokine signalling. This suggests that although zebrafish cannot recapitulate the cellular and physiological diversity of human adaptive immune system, the early innate responses to disease can have parallels.
Our study showed interesting results that the molecular pathways and genes altered in astrocytes after amyloid toxicity have significant differences as well as meaningful similarities in humans and zebrafish. In both organisms, astrocytes regulate toxic protein clearance, unfolded protein response and energy metabolism, while zebrafish and humans differentially regulate pathways including glutamate clearance, lipid metabolism, cell proliferation and neurogenesis. Particularly, zebrafish astrocytes turn on mechanisms that mediate formation of new neurons while human astrocytes fail to do so. A pathological outcome of Alzheimer’s disease is reduced neurogenesis. Provided that neurogenesis could be enhanced in the human brains, we might cope better with neurodegeneration via a more resilient neuronal circuitry. However, the molecular mechanisms governing pathology-induced neurogenesis are not fully known.
|
Figure 2. Transition analysis between cell clusters. (A) tSNE plot showing the main cell types in zebrafish when all genes annotated in zebrafish are used for clustering. (B) tSNE plot showing the main cell types in zebrafish when only the genes orthologous to humans are used for clustering. (C) Transition diagram between (A,B). When human orthologous genes are used, majority of the cell types remain in their clusters, with slight exception of a subset of oligodendrocytes, excitatory neurons, and inhibitory neurons that start clustering in astroglia. (D) tSNE plot showing the main cell types in humans when all genes annotated in humans are used for clustering. (E) tSNE plot showing the main cell types in humans when only the genes orthologous to zebrafish are used for clustering. (F) Transition diagram between (D,E). When zebrafish orthologous genes are used, the vast majority of the cell types remain in their clusters.
|
Therefore, the current study, in combination with our previous studies, suggests that the uniquely altered pathways in zebrafish astrocytes could highlight the neurogenesis mechanisms absent in humans in Alzheimer’s disease. These mechanisms could serve as potential candidates for therapeutic avenues for enhancing the endogenous neurogenic outcome in human brains via inspiration from the nature. A potential continuation of our study is to investigate the effects of zebrafish neurogenic programs in mammalian brains in health and disease, and to determine whether enhanced neurogenesis could alter Alzheimer’s disease pathology.
Caghan Kizil, PhD
Visiting Professor of Neurological Sciences (in Neurology)
ck2893@cumc.columbia.edu
Giuseppe Tosto, MD, PhD
Assistant Professor of Neurological Sciences (in Neurology, the Taub Institute for Research on Alzheimer's Disease and the Aging Brain and the Gertrude H. Sergievsky Center)
gt2260@cumc.columbia.edu