Columbia University
Irving Medical Center
Neurological Institute
710 West 168th Street, 3rd floor
(212) 305-1818
TaubCONNECT Research Perspectives:
January 2022
2: Rab35 and Glucocorticoids Regulate APP and BACE1 Trafficking to Modulate Aβ Production
3: The Neuronal Retromer can Regulate Both Neuronal and Microglial Phenotypes of Alzheimer's Disease
4: Deep Learning Improves Utility of Tau PET in the Study of Alzheimer's Disease
 |  |
| Dolly Reyes-Dumeyer | Richard Mayeux, MD, MSc |
The National Institute on Aging Late-Onset Alzheimer's Disease Family Based Study (NIA-LOAD FBS) is the largest collection of multiplex Alzheimerās disease (AD) families recruited and longitudinally assessed worldwide. Since its inception in 2003, the central goal of this cohort has been to broadly support genetic research by making clinical and genomic data and biological samples rapidly available to the AD research community, to support the development of therapies to treat or prevent AD. In the current issue of Alzheimerās & Dementia, the NIA-LOAD FBS collaborative, including the studyās invaluable project manager, first author Dolly Reyes-Dumeyer, describes the cohort and highlights the broad availability of clinical and genetic data available to the AD research community.
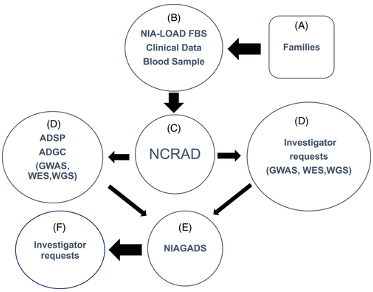
Figure 1. Potentially qualifying families (A) were recruited by the National Institute on Aging Late-Onset Alzheimer's Disease Family Based Study (NIA-LOAD FBS) investigators (B). Blood was sent to National Centralized Repository for Alzheimer's Disease and Related Dementias (NCRAD) (C). NCRAD supplied biological samples to the Alzheimer's Disease Sequencing Projects (ADSP) (D) and to other qualified individual investigators (D) along with phenotype data from the Columbia University Coordinating Center. Any new data generated by investigators was required to be shared by sending it to National Institute on Aging Genetics of Alzheimer's Disease Data Storage Site (NIAGADS; E) where other investigators could request clinical and genetic results from these studies (F).
To date, we have recruited 1756 diverse familiesā181 (10.3%) Black; 425 (24.2%) Caribbean Hispanic; 138 (7.8%) self-reporting as āother or mixedā; and 1012 White, non-Hispanicāand acquired data from 9682 additional family members. Among the 1756 families, 451 (25.7%) had three or more affected family members. Another 31.5% had two affected individuals while 598 (34%) families consisted of only a single affected individual or a single individual who, after clinical evaluation, did not meet criteria for dementia or AD but had cognitive impairment.
Uniform assessments and DNA were obtained from all families, and apolipoprotein E (APOE) genotypes, genome-wide single nucleotide polymorphism (SNP) arrays, and whole exome and whole genome sequencing has been completed in most families. Neuropathological examinations have been conducted in 836 individuals: AD was confirmed in 492 (82%) of these participants, and other diagnoses accounted for the remaining 163, with and without dementia. In some large families, the age-at-onset varied by as much 22 years suggesting the possibility of genetic or environmental modifying factors. All biological resources and acquired data, including brain samples, are available to be shared with researchers via the National Centralized Repository for Alzheimer's Disease and Related Dementias (NCRAD).
Going forward, we intend to expand capacity for multiomics research, include recently developed blood-based biomarkers to add precision to the diagnoses, and expand the ethnic diversity of families recruited. Importantly, as of June 2022, we will be broadening our focus to include early-onset AD families, and will thenceforth be known as the NIA-AD FBS.
Dolly Reyes-Dumeyer
Senior Staff Associate II
Taub Institute for Research on Alzheimerās Disease and the Aging Brain
dr2290@cumc.columbia.edu
Richard Mayeux, MD, MSc
Gertrude H. Sergievsky Professor of Neurology, Psychiatry and Epidemiology (in the Sergievsky Center and Taub Institute); Chair, Dept, of Neurology; Director, Sergievsky Center; Co-Director, Taub Institute
rpm2@cumc.columbia.edu
Rab35 and Glucocorticoids Regulate APP and BACE1 Trafficking to Modulate Aβ Production
 |  |
|
| Viktoriya Zhuravleva, PhD | Clarissa Waites, PhD |
Chronic stress and elevated levels of glucocorticoids (GCs), the major stress hormones, are risk factors for Alzheimerās disease and promote AD-like pathology in animal models. Indeed, both the overproduction of toxic amyloid-β (Aβ) peptides and the intraneuronal accumulation of hyperphosphorylated Tau protein are induced by chronic stress paradigms in rodents. How these AD-related pathologies are triggered by stress/GCs at the cellular level remains poorly understood.
In a previous study, we uncovered a mechanism by which stress/GCs promote Tau pathology in hippocampal neurons. Specifically, we found that GCs transcriptionally downregulate Rab35, a small GTPase that stimulates the clearance and degradation of Tau. By turning down Rab35 expression, GCs promote Tau accumulation, leading to its phosphorylation, missorting to synapses, and ultimately to downstream synapse and dendrite loss. Since Rab35 is considered a 'master regulator' of intracellular protein trafficking, we wondered whether its downregulation could also trigger stress/GC-induced Aβ peptide overproduction.
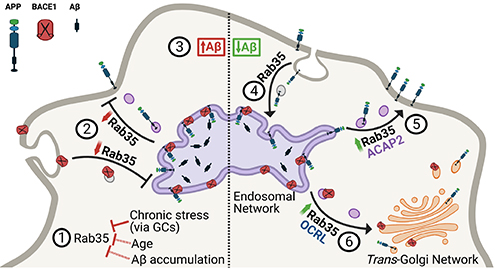
Figure 7. Working model summarizing the interplay between Rab35 and stress/GC on APP trafficking and processing. View full caption.
In the current study, published in Cell Death & Disease, we perform a combination of in vivo and in vitro experiments to investigate whether and how Rab35 regulates Aβ production. Interestingly, we find that Rab35 levels in the rodent hippocampus decrease not only in response to stress, but also with aging, another AD risk factor. Moreover, we show that overexpression of Rab35 in the hippocampus of aged rats (via adeno-associated virus) prevents the processing of amyloid precursor protein (APP) toward Aβ, indicating a protective effect of Rab35. Our in vitro experiments further confirm this effect, showing that Rab35 inhibits Ab production by regulating the intracellular trafficking of both APP and b-secretase1 (BACE1), which mediates the rate-limiting cleavage of APP into Aβ. In particular, we find that Rab35 mediates distinct trafficking events for APP and BACE1 via distinct molecular pathways, ultimately resulting in fewer APP/BACE1 interactions within the endosomal network (and thereby less Aβ production). These findings suggest that Rab35 is protective against Aβ production, and that its downregulation by stress/GCs may upregulate amyloidogenesis. Supporting this concept, we observe that high GC levels alter APP and BACE1 trafficking to increase their interaction in endosomes (thus stimulating Aβ production), and that overexpression of Rab35 counteracts these effects. Together, our work supports multiple roles for Rab35 in the trafficking of AD-relevant proteins, and indicates that its downregulation is a precipitating factor in stress/GC-induced amyloid and Tau pathology. Further investigation of Rab35-related trafficking pathways could open novel therapeutic avenues for treating AD.
Clarissa Waites, PhD
Associate Professor of Pathology and Cell Biology and Neuroscience
Taub Institute for Research on Alzheimerās Disease and the Aging Brain
cw2622@cumc.columbia.edu
The Neuronal Retromer can Regulate Both Neuronal and Microglial Phenotypes of Alzheimer's Disease
 |  | |
| Yasir H. Qureshi, MD | Scott Small, MD |
The link between endosomal recycling and AD-associated pathologies has been shown by a range of studies that have manipulated retromer in different model systems (Small and Petsko, 2015). To date, however, most in vivo mouse studies have been performed in transgenic mice engineered to overexpress human mutations in APP and PSEN1/2 (Wen et al., 2011, Li et al., 2019), or FTD-associated mutations in the tau gene MAPT (Vagnozzi et al., 2019, Carosi et al., 2020). While these studies are useful, they do not model the common āsporadicā form of AD, in which these mutations do not occur.
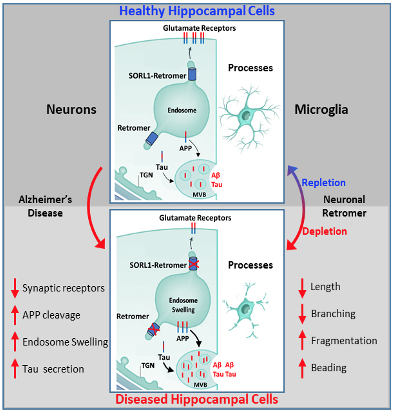
Figure 1. Schematic representation of endososomal recycling system in neurons and its effects on microglia. Upper panel shows a normal neuron with healthy endosomal/retromer recycling of glutamate receptors, APP and Tau with a healthy microglial population accompanying it. Lower panel shows AD neuron with defective endosomal trafficking resulting in endosomal swelling, APP and tau misprocessing and degradation of glutamate reseptors. This results in impared secretion from the cell and in turn causes dystrophic changes in microglia. Similar changes were observed in mice when endosomal trafficking was disrupted in neurons.
In the current study, recently published in Cell Reports, we used a mouse model of endosomal dysfunction to investigate AD phenotypes in the absence of APP, tau, or PSEN mutations. The cortical and hippocampal neurons in these mice were specifically deficient in a key regulator of endosomal trafficking pathway, and a member of the retromer core complex; the VPS35 protein. This VPS35 deficiency resulted in disruption of the retromer core complex in neurons and caused endosomal trafficking deficits leading to APP misprocessing, aberrant Glutamate receptor trafficking, and SORL1 accumulation in the endolysosomal system. In addition to this and to our surprise we saw a remarkable phenotype in the glial cell population in these animals. Further investigation revealed that the microglia in this model morphologically phenocopy the dysmorphic microglia observed in AD patients (Navarro et al., 2018, Davies et al., 2017, Tischer et al., 2016, Shahidehpour et al., 2021). In fact, these dystrophic microglia are diametrically distinct from hypertrophic microglia, which are characterized by an exuberant overgrowth of microglia processes. We used AAV vectors to replete VPS35 in hippocampus of these mice, and were able to normalize the AD phenotypes, both in the neurons and glia, further validating their association with endosomal trafficking.
More importantly these neuronal and glial phenotypes were resistant to knocking out tau. This supports the hypothesis that ADās neuronal and microglia pathology might occur independent of, and in parallel to, tau pathology (Small and Petsko, 2020). The neuronal-selective retromer depleted model also instantiate a cellular crosstalk between neurons, the cell type where AD begins, with microglia, a cell type that is implicated in AD genetic risk by modulating disease onset and progression. This cellular dialogue is now considered at the nexus of AD pathophysiology. The current study sets the stage for future studies to isolate the specific mechanisms by which retromer depletion in neurons, signals to microglia, astrocytes and other non-neuronal cells.
Yasir H. Qureshi, MD
Associate Research Scientist
Taub Institute for Research on Alzheimerās Disease and the Aging Brain
yhq2001@cumc.columbia.edu
Scott Small, MD
Boris and Rose Katz Professor of Neurology
Director, Alzheimer's Disease Research Center
Taub Institute for Research on Alzheimer's Disease and the Aging Brain
sas68@cumc.columbia.edu
Deep Learning Improves Utility of Tau PET in the Study of Alzheimer's Disease
 |  | |
| James Zou, MD (VP&S 2021) | Frank Provenzano, PhD |
Positron emission tomography (PET) imaging targeting neurofibrillary tau tangles has become a valuable tool for mapping and quantifying tau burden in studies of Alzheimerās disease (AD). Unlike metabolic PET imaging, which uses the same analog of glucose, 18-FDG, there is a growing array of tau PET tracers and acquisition criteria that contribute to the heterogeneity and validity of cohesive estimates of tau in the brain.
In a new study in Alzheimerās & Dementia: DADM, we sought to examine this problem by developing, training, and evaluating deep learning models of tau PET imaging to predict impairment using only the participantās tau PET scan, among a range of healthy and impaired individuals. Using tau PET images (18FāMK6240 (n = 320) and AVā1451 (n = 446)) pooled from multiple studiesāincluding studies from Taub faculty members Drs. Kreisl, Brickman, Luchsinger, and Devanand, as well as ADNIāwe created a convolutional neural networks (CNNs) framework representing two PET radioligands from different generations, with highly similar functional binding.
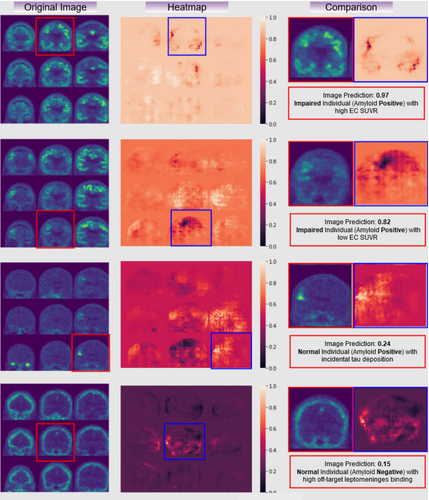
Figure 3. Examples of raw tau PET images (left column), occlusion sensitivity analyses examining the result of the classification (middle), and a comparison of the two with the subject status (right). Here, you can see different areas of signal where cases without clear raw tau PET findings, like the 2nd and 3rd row, might be aided by the use of a CNN network.
With this framework, we were able to match or exceed predicted memory impairment accuracy when compared to a gold standard standard entorhinal cortex region-of-interest (ROI) analysis alone, agnostic of clinical or amyloid status. Although performances varied depending on which model was applied to which radioligand, all AUC (area under the curve) outperformed ROI analysis, with the highest AUC reported to be AUC (0.89 vs. 0.72, Z = 5.2, P < .0001) of both ligands applied to 18F-AV-1451 data. This study shows that deep learning CNNs (our lab has made the CNN used in this paper publicly available), which are known to offer high accuracy despite noisy or diverse training data, can be applied for classification tasks with minimal processing to tau PET, and are especially beneficial in cases of early or prodromal disease, where target binding signal might not be particularly robust.
Frank Provenzano, PhD
Assistant Professor of Neurological Sciences Assistant Professor of Neurological Sciences (in Neurology and in the Taub Institute for Research on Alzheimer's Disease and the Aging Brain)
fap2005@cumc.columbia.edu

