Columbia University
Irving Medical Center
Neurological Institute
710 West 168th Street, 3rd floor
(212) 305-1818
TaubCONNECT Research Perspectives:
April 2021
2: Complexity and Graded Regulation of Neuronal Cell-Type-Specific Alternative Splicing Revealed by Single-Cell RNA Sequencing
3: The Microtubule Cytoskeleton at the Synapse & The Synaptic Life of Microtubules
4: Distinct Cortical Thickness Patterns Link Disparate Cerebral Cortex Regions to Select Mobility Domains
 |  | |
| Reena T. Gottesman, MD | Yian Gu, MD, MS, PhD |
Neuropsychiatric symptoms are an important feature of Alzheimer’s Disease (AD), as well as AD management, contributing to caregiver stress and increased medical resource use and costs. In epidemiologic studies, psychotic symptoms, such as hallucinations and delusions, have been demonstrated to predict a faster progression to endpoints of cognitive and functional decline. Much of this research has been previously conducted in Caucasian clinic-based cohorts, which limits generalizability to diverse populations. In a new study based on the Predictors 3 cohort, which includes predominantly Hispanic AD patients identified from the community-based Washington Heights-Inwood Columbia Aging Project (WHICAP), Drs. Reena Gottesman and Yian Gu, along with colleagues, sought to confirm whether these neuropsychiatric predictors of progression would apply in a community-based cohort with minimal educational attainment.
As recently reported in the Journal of Alzheimer’s Disease, they found that those with psychotic symptoms were at baseline more impaired cognitively and functionally compared to those without psychosis. The presence of psychotic symptoms at baseline predicted functional decline and dependency; however, this association was mitigated when taking baseline function into account. These results suggest that prognostic factors in AD may differ among different populations and highlight the need for community-based research.
Reena T. Gottesman, MD
Assistant Professor of Neurology
rtg2120@cumc.columbia.edu
Yian Gu, MD, MS, PhD
Assistant Professor of Neurological Sciences (in Neurology, Epidemiology and the Taub Institute)
yg2121@cumc.columbia.edu

Vilas Menon, PhD
An important question in addressing cellular diversity in the mammalian brain is developing a catalog of cell types defined by their gene and protein expression profiles. In recent years, single-cell RNA-sequencing has become an invaluable tool to address this diversity with high resolution. However, the majority of these studies are not powered enough to address cell type-specific splicing differences, which have important consequences for protein composition and function of cell types. Here, Taub Faculty Dr. Vilas Menon paired with Dr. Chaolin Zhang, a faculty member in the Columbia Motor Neuron Center, to reanalyze single-cell RNA-seq data from adult mouse cortex. In particular, they investigated how regulation of alternative splicing differs among the multitude of transcriptomically-identified cell types identified by Dr. Menon and colleagues in a previous study.
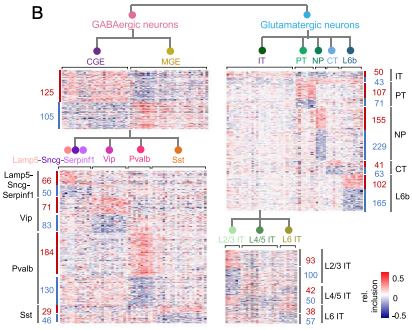
Figure: Heatmaps showing relative exon inclusion level (with median subtraction) of DS exons between neuronal subclasses detected at different hierarchical levels. Each row represents an exon and each column represents a cell type. The number of DS exons in each comparison is labeled.
Given that different neuronal subtypes have distinct morphologies, connectivity, and function, a comprehensive understanding of their alternative splicing regulation would lay the foundation for investigating dysregulation in the context of neurological disease. Together, their analysis uncovered distinct splicing programs between excitatory and inhibitory neurons, as well as among key subclasses within each neuronal class, as recently reported in PNAS. In particular, it appears that RNA-binding proteins (RBPs) Celf1/2, Mbnl2, and Khdrbs3 are preferentially expressed and more active in glutamatergic neurons, while Elavl2 and Qk are preferentially expressed and more active in GABAergic neurons. Because these classes of neurons and their corresponding subtypes have been found to be differentially susceptible in Alzheimer’s disease and other aging-associated neurological conditions, identifying these general patterns of alternative splicing highlights potential pathways underlying resistance and vulnerability, an important theme of Dr. Menon’s research at the Taub Institute.
Vilas Menon, PhD
Assistant Professor of Neurological Sciences (in Neurology and the Taub Institute for Research on Alzheimer Disease and the Aging Brain)
Center for Translational and Computational Neuroimmunology (CTCN)
vm2545@cumc.columbia.edu
The Microtubule Cytoskeleton at the Synapse & The Synaptic Life of Microtubules
 |  |  | ||
| Francesca Bartolini, PhD | Xiaoyi Qu, PhD | Clarissa Waites, PhD |
Neurons possess both stable and dynamic microtubules composed of the polarized addition of α- and β-tubulin subunits with free minus and plus ends. Dynamic microtubules differ from stable microtubules in their ability to undergo stochastic transitions between polymerization and depolymerization, and neuronal homeostasis depends on the balance between pools of stable and dynamic microtubules. While it has long been known that stable microtubules support the structure of axons and dendrites, the roles of microtubules at synapses have been explored only over the past decade. Here, two recent papers by Taub affiliate Dr. Francesca Bartolini review emerging literature demonstrating that microtubules enter both excitatory and inhibitory synapses and control central aspects of synaptic activity, including neurotransmitter release and synaptic plasticity. Multiple labs have now reported that dendritic spines are invaded by dynamic microtubules depending on synaptic activity, Ca2+ influx and actin dynamics, and that microtubule invasion regulates spine enlargement and synaptic strength by restricting the delivery of selected cargos.
The role of microtubules at mammalian presynaptic elements has, in contrast, remained uncharted territory until very recently. In highly active synapses that require accurate and graded neurotransmitter release, such as ribbon synapses in the Calyx of Held, presynaptic microtubules are rate limiting for high-frequency neurotransmission by replenishing the readily releasable pool of synaptic vesicles from the reserve pool, and in anchoring mitochondria at sites of high metabolic demand. In hippocampal axons, en passant boutons are enriched in dynamic microtubules that allow for the targeted delivery and unloading of synaptic vesicle precursors by the kinesin-3 motor, KIF1A. The Bartolini lab, in collaboration with Dr. Clarissa Waites, has extended these observations by demonstrating that excitatory boutons are hotspots for activity-evoked microtubule nucleation, and that this newly formed subset of dynamic microtubules regulates neurotransmission by providing tracks for bidirectional interbouton delivery of a rate-limiting supply of synaptic vesicles. Whether regulation of synaptic microtubules is affected in neurological disease remains completely unknown and an exciting new area of investigation.
Francesca Bartolini, PhD
Assistant Professor of Pathology and Cell Biology at the Columbia University Medical Center
fb2131@cumc.columbia.edu
 |  | |
| Yaakov Stern, PhD | Christian Habeck, PhD |
In the past, two common problems associated with aging, falls and dementia, were viewed as part of parallel, unrelated worlds. Today, a growing body of literature has provided associations between gait, cognitive decline, falls, and dementia, but the underlying neural mechanisms are not well understood. Together with Tel Aviv University colleagues and movement experts Drs. Inbal Maidan, Anat Mirelman, and Jeffrey Hausdorff, Taub faculty members Drs. Yaakov Stern and Christian Habeck investigated the association between regional cortical thickness and gait in 115 healthy participants, aged 27-82, in a study recently published in Scientific Reports. Their results show lower mean cortical thickness to be associated with worse mobility and gait. Moreover, specific associations were identified between distinct spatial patterns of cortical thickness and three relatively simple gait domains: pace, rhythm, and symmetry. Interestingly, no associations were found between cortical thickness and more complex mobility domains.
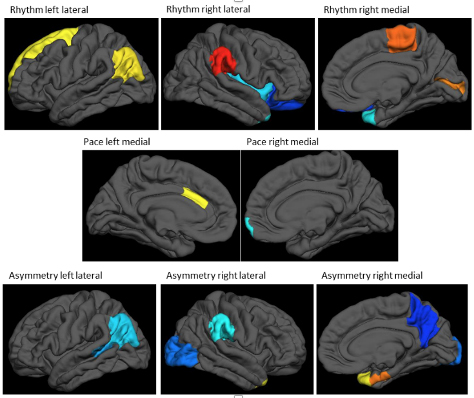
Figure: cortical thickness patterns associated with 3 different gait measures (rhythm, pace, asymmetry). Warm colors indicate a positive association of cortical thickness with the gait measure, while cold colors indicate negative associations. The analytical framework removes mean cortical thickness, such that people with higher gait scores will have relatively thicker cortices at the regions indicated with warm colors, and relatively thinner cortices (relative to their whole-brain mean thickness) at cold-colored regions. (Figure adapted and edited from primary article.)
These findings indicate that distinct regional patterns of cortical thickness determine the basic components of gait. However, once the complexity of mobility increases, additional factors come into play. It is important to emphasize that this study investigated brain-gait-cognition relations using only measures of brain structure. Features of brain function, like regional neural connectivity and activation, were not considered, but might enable a more complete understanding of the interaction between cognition and mobility. The mechanistic underpinnings and predictive utility of regional activation will be explored in future joint projects.
Christian Habeck, PhD
Professor of Neurological Sciences (in Neurology and in the Taub Institute) at the Columbia University Medical Center
ch629@cumc.columbia.edu

