Columbia University
Irving Medical Center
Neurological Institute
710 West 168th Street, 3rd floor
(212) 305-1818
Featured Research
In the Lab:
William C. Kreisl, MD

William C. Kreisl, MD
As a clinician with significant expertise in molecular imaging research, my goal is to identify critically-needed molecular targets for improved diagnosis and treatment of Alzheimer's disease (AD) and other neurodegenerative disorders. Advances in positron emission tomography (PET) imaging and cerebrospinal fluid (CSF) biomarker analysis have demonstrated the potential of these methodologies for validating drug targets and quantifying disease progression and treatment response. Research in my clinical neuroscience laboratory focuses on the use of these methodologies to determine pathogenic processes underlying diseases of aging, to inform clinical trials of novel disease-modifying drugs. While amyloid plaques are a pathological hallmark of AD, up to 30% of cognitively normal elders are amyloid-positive on PET. This discrepancy suggests that other pathogenic factors are required for cognitive decline in the setting of amyloidosis. Thus, my primary research aim is to determine the role of inflammation and tau pathology in cognitive impairment, in the presence and absence of amyloid.
Most of my research career, thus far, has been focused on translating use of novel PET radioligands into clinical application in neurodegenerative diseases. Specifically, I focused on developing radioligands for accurate in vivo measurement of two protein targets: translocator protein (TSPO) 18kDa, a marker for inflammation, and Permeability-glycoprotein (P-gp), an efflux transporter at the blood-brain barrier. I have published studies in non-human primates, healthy human volunteers, and patients with Alzheimer's disease evaluating these PET ligands.
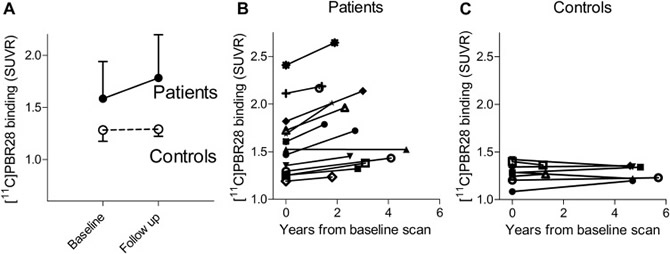 Figure 1. Translocator protein 18 kDa (TSPO) increases over time in patients with Alzheimer's disease (AD) and mild cognitive impairment (MCI) (‚óŹ) but not in older controls (‚óč). (A) 11C-PBR28 binding to TSPO (standardized uptake value ratio [SUVR]) at baseline and follow-up (median interval 2.7 years) is shown for inferior parietal lobule. Data are presented as mean ¬Ī SD. Patients (B) showed variable annual increases in TSPO binding, whereas controls (C) showed no increased binding despite longer follow-up intervals. For (B) and (C), 11C-PBR28 binding in inferior parietal lobule is shown for individual subjects (symbols). Abbreviation: SD, standard deviation. |
Among my findings related to TSPO, I have shown that patients with AD have greater binding of the second generation TSPO radioligand 11C-PBR28 than patients with mild cognitive impairment or healthy controls, and that binding correlates with clinical severity, the amount of brain atrophy on MRI, and younger age of onset. I later oversaw the validation of a ratio method for 11C-PBR28 analysis in AD that reduces variance, removes need for arterial sampling, and shortens scan time, thus reducing the number of subjects needed for clinical studies and improving subject tolerability. These and other contributions are expected to make 11C-PBR28 imaging more accessible for clinical PET studies. Use of 11C-PBR28 PET, in turn, is expected to be useful for tracking progression of AD and monitoring response to novel treatments.
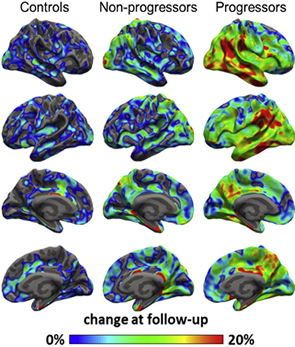 Figure 2. Average percent change in 11C-PBR28 binding from baseline, overlaid on semi inflated cortical surface. Patients who showed clinical progression during the study interval (n = 9) had greater increases in 11C-PBR28 binding than nonprogressors (n = 5) or controls (n = 8), with the greatest change observed in inferior temporal and parietal cortices.;
Figure 2. Average percent change in 11C-PBR28 binding from baseline, overlaid on semi inflated cortical surface. Patients who showed clinical progression during the study interval (n = 9) had greater increases in 11C-PBR28 binding than nonprogressors (n = 5) or controls (n = 8), with the greatest change observed in inferior temporal and parietal cortices.;In addition to the above contributions, I helped develop the radiotracer 11C-N-desmethyl-loperamide (dLop) to measure the function of P-gp at the human blood-brain barrier, demonstrating that the radioligand was not only safe for human use, but that it did not enter brain under normal circumstances due to efflux activity of P-gp. I extended this work to show that P-gp function could be quantified in human brain using dLop and the specific P-gp inhibitor tariquidar. These and other related contributions provide an optimal method of measuring P-gp function at the human blood-brain barrier in living subjects.
Currently, in collaboration with several colleagues and mentors at CUMC, including Taub faculty members Drs. Richard Mayeux, Yaakov Stern, and Lawrence Honig, my laboratory is focused on extending this neuroimaging research to determine 1) how inflammation and tau each relate to amyloidosis, neurodegeneration, and cognition, and 2) the spatial relationship among inflammation, tau, and amyloid. This is a first step toward my long-term goal of developing neuroimaging strategies to predict future cognitive decline in elderly individuals, and inform pharmacological studies targeting inflammation and tau pathology in AD.
William C. Kreisl, MD
Boris and Rose Katz Assistant Professor of Neurology (in the Taub Institute)
wck2107@cumc.columbia.edu

