Columbia University
Irving Medical Center
Neurological Institute
710 West 168th Street, 3rd floor
(212) 305-1818
Featured Research
IN THE LAB:
Andrew Teich, MD, PhD
 Andrew F. Teich, MD, PhD
Andrew F. Teich, MD, PhDThe fields of computer science and molecular biology are arriving at a point where they can be combined to explore how genes influence disease. Research in my laboratory is focused on using this new synergy to understand how neurons communicate, and how this communication is disrupted in Alzheimer's disease (AD). In order for memory formation to work, neurons need to turn on genes that strengthen communication. This process is regulated by signaling molecules that precisely determine when genes should be turned on and off. In the same way that a conductor conducts an orchestra, these signaling molecules coordinate the genome, so that the resulting read-out of a cell's genome makes sense (music) instead of chaos (random noise). We have evidence that in AD, one of the reasons people have problems forming memories is that the signaling molecules in neurons are dysfunctional. In other words, something is wrong with the conductors, so the genomic orchestra is producing chaos. One of the big problems in the neuroscience field right now is that we don't yet know who all the conductors are. The first goal of my research is to use the synergy between computer science and molecular biology to catalog all of the conductors (signaling molecules) that are coordinating the genes that are important for strengthening neuronal communication. In parallel, I am also examining what happens to the conductors during memory impairment in AD. Working with me on all of these projects is Postdoctoral Research Scientist Zeljko Tomljanovic.
To do this work correctly requires, first, access to carefully selected brain tissue from patients at various stages of disease, and second, extensive collaboration. For example, one of our most innovative and exciting projects is a collaboration with Dr. Guy McKhann, from the Department of Neurosurgery, to examine how genes are being aberrantly turned on and off in the earliest stages of AD. This project involves going into the Operating Room and collecting small biopsies of cortex that are routinely removed for hydrocephalus surgery (a brain condition unrelated to Alzheimer's disease). However, because hydrocephalus patients are often relatively advanced in age, some of these patients have findings in their brain tissue and fluids that suggest they may be at the earliest stages of AD. We screen brain tissue from these patients using advanced sequencing technologies to determine which genes are being aberrantly turned on and off, and we correlate this with various biomarkers that relate to Alzheimer's disease progression. In this effort, we also collaborate with Dr. Peter Sims in the Department of Systems Biology at Columbia University, who is assisting with additional computational details.
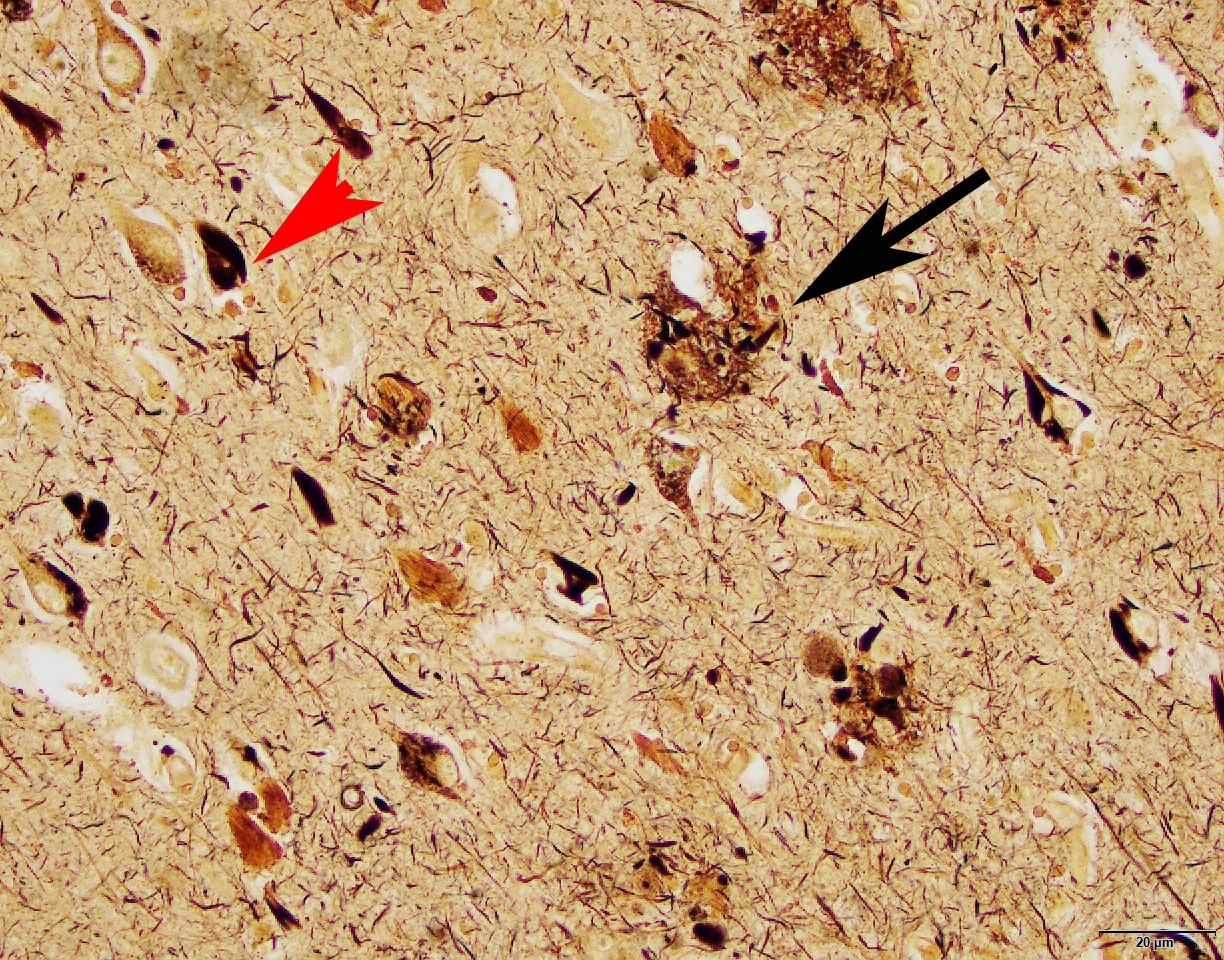 A microscopic view of brain tissue from a patient with Alzheimer's disease. Beta-amyloid plaques (black arrow) and neurofibrillary tangles (red arrow head) are the hallmarks of the disease. New sequencing technologies are allowing scientists like Dr. Teich to untangle the molecular pathways that are disrupted in Alzheimer's disease brain tissue.
|
A second project has come out of a collaboration with Drs. Andrea Califano (Department of Systems Biology, Biochemistry & Molecular Biophysics, and Biomedical Informatics at Columbia University Medical Center) and Michael Shelanski (Department of Pathology and Cell Biology and the Taub Institute). In this project, data from a large collection of brains at varying stages of AD is being combined to determine which signaling molecules are primarily responsible for aberrantly turning on or off large groups of genes. This is of interest because these signaling molecules (called "master regulators") may explain a great deal of the disrupted gene function in AD, and as such, represent prime therapeutic targets. My laboratory has further screened this data to arrive at a number of master regulators whose dysfunction may be causing neurons to communicate aberrantly. This is particularly exciting, because these candidates may be linked to the dementia of Alzheimer's disease in a rather direct way. A top candidate from this analysis is currently being intensively studied in my laboratory, and may represent a novel way to address the dementia of Alzheimer's disease.
 Members of the Andrew Teich Laboratory include, from left to right, Felix Rozenberg, Andrew Teich, and Zeljko Tomljanovic.
|
As my primary lab member, Zeljko Tomljanovic works to coordinate projects with various volunteers who rotate through my laboratory, in addition to his work on the projects highlighted above.
Felix Rozenberg is an undergraduate at Columbia who volunteers his time to work on several ongoing collaborations between my lab and the lab of Dr. Ottavio Arancio; the goal of this work is to better understand the physiologic role of beta-amyloid, which is one of the primary disease causing proteins in AD.

