Columbia University
Irving Medical Center
Neurological Institute
710 West 168th Street, 3rd floor
(212) 305-1818
Featured Research
In the Lab:
Sabrina Simoes, PhD

Sabrina Simoes, PhD
Dr. Sabrina Simoes is a neurobiologist who has spent her career studying endosomal trafficking in normal and pathological conditions. Currently, research in her lab focuses on understanding the mechanisms underlying the pathogenesis of Alzheimer’s disease (AD), with special emphasis on endo-lysosomal dysfunction. During her undergraduate and graduate training in the Curie Institute laboratory of Dr. Graca Raposo—a world leader in the exosome/endosome field—she developed a strong interest in intracellular trafficking. Her early studies focused on the mechanism by which prion proteins could spread within an organism and among cells. At the time, it was proposed that prions could exploit exosomes for their spreading in vivo, but the underlying mechanisms were poorly understood. This question became the main goal of her Master’s thesis, which focused on 1) understanding how prion proteins could be targeted to exosomes in multivesicular endosomes, and 2) identifying the endosomal trafficking machineries involved in this process. In collaboration with various investigators, she completed her thesis showing that, in addition to exosomes, retroviral particles could contribute to prion spreading in vitro, (Figure 1, upper left panel).
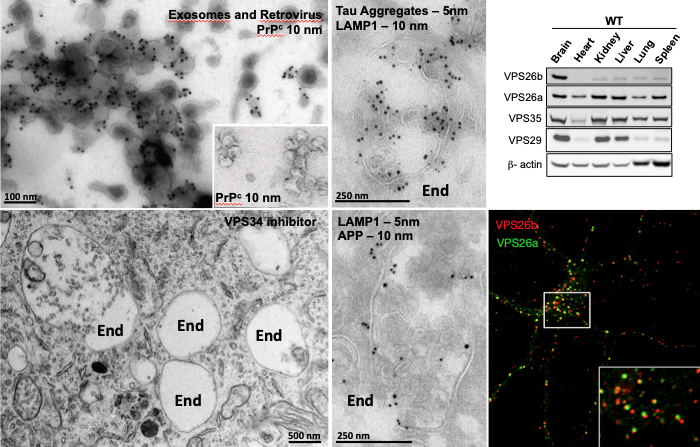 Figure 1: Cryo Immuno-gold Electron Microscopy (IEM) of purified exosomes and retroviral particles immuno-labeled for the Prion Protein, PrPc (upper left panel). Conventional EM showing enlarged endosomes in cell treated with an inhibitor of VPS34 protein. End, Endosome (lower left panel). Examples of cryo-immunogold labeling of endosomal structures double labeled for APP, Tau aggregates and LAMP1 (two middle panels). Western blot analysis showing that while VPS26a is ubiquitously expressed among all tissues, VPS26b is highly enriched in the brain (upper right panel). Confocal microscopy showing partial intracellular overlap of both VPS26 paralogs in primary hippocampal cultures (lower right panel).
|
During her PhD, she continued along this research line, focusing on how ubiquitous mechanisms of endosomal sorting and intracellular trafficking could underlie the biogenesis of endosome-derived organelles, called melanosomes. To this aim, she explored the function of various endosomal machineries including, the Endosomal Sorting Complexes Required for Transport (ESCRTs) and Tetraspanins, in the sorting of endosomal cargoes required for the generation of these organelles. This work contributed to the identification of a new endosomal sorting machinery within endosomes that sorts cargo through an independent ESCRT mechanism.
In 2011, she joined the Taub Institute laboratory of Dr. Scott Small, where she continued to pursue her studies on endosomal trafficking in the context of neurodegeneration. In recent years, dysfunction of the endo-lysosomal network has gained considerable attention in the neurodegeneration field and has been linked to several neurological diseases. For instance, genetic and cell biological findings in AD have implicated ‘endosomal trafficking’ as playing a central role in the disease. Some of the best evidence in support of this conclusion is provided by molecules related to retromer, a multi-protein assembly that functions in sorting and trafficking of cargo out of the endosome, back to the Trans-Golgi Network (retrograde pathway) or the plasma membrane (recycling pathway). In a study, recently presented at the Neurology Research Retreat, Dr. Simoes demonstrated that dysfunction of VPS26b—a retromer core component highly expressed in the brain dedicated to endosomal recycling (Figure 1, right panels)—specifically targets the trans-entorhinal cortex, a region within the brain most vulnerable to AD. Additionally, she contributed to the identification of a new family of small molecules, retromer pharmacological chaperones, which were shown to stabilize the interaction between members of the retromer complex, and to enhance its function in neurons. These molecules were shown to improve retromer trafficking and endosomal dysfunction, with significant therapeutic potential for neurological disorders.
Since joining the Taub Institute, Dr. Simoes has applied both her unique expertise in several Electron Microscopy (EM) techniques and her advanced knowledge of the endolysosomal system to establish several fruitful collaborations with a large number of investigators, including Drs. Karen E. Duff and Gilbert di Paolo (now at Denali Therapeutics, Inc.) (Figure 1). She has co-authored studies aimed at investigating the intracellular trafficking of APP; examining the endosomal uptake of tau aggregates by different cell types; and, more recently, looking at endosomal dysfunction and atypical exosomal secretion in cell lines and mouse models. Currently, Dr. Simoes is collaborating with Dr. Catherine Marquer to investigate inter-organelle contact sites using cryo-immunogold labelling and electron tomography. In another collaborative study with Drs. Richard Mayeux and Andrew Sproul, she is looking into endosomal dysfunction in SorLa mutant and isogenic control human iPSC-derived neurons by confocal and conventional EM.
 Figure 2: Quanterix SR-X Ultra-Sensitive Biomarker Detection System (left panel), Meso Quickplex SQ 120 instrument (middle panel) and Nanotracker Zetaview PMX 120S (right panel).
|
In her most recent mouse-to-human study (Simoes S. and Neufeld J. et al., under revision in STM), two potential biomarkers of endosomal dysfunction for AD have been identified. Candidates were initially found in a mouse model showing endosomal trafficking defects, and further validated in prodromal AD patients using Single Molecule Array (Simoa) technology (Figure 2). Currently, Dr. Simoes has a number of ongoing collaborations aimed at measuring AD biomarkers in CSF and plasma/serum samples in mouse and rat models (Drs. Adam Brickman and Francesco Lotti) and human subjects (Drs. Lawrence S. Honig, Frank Provenzano, William C. Kreisl and Sarah E. Tom). Going forward, she aims to identify and develop new peripheral biomarkers (including brain-derived exosomes) for AD and other neurodegenerative disorders.
Dr. Simoes was recently promoted to assistant professor of neurological sciences (in Neurology and the Taub Institute) and recruited to the Alzheimer’s Disease Research Center (ADRC), where she will use Simoa, Mesoscale (MSD) Multi-Array Technology, and a nanoparticles tracking system for biomarker studies (Figure 2).
Sabrina Simoes, PhD
Assistant Professor of Neurological Sciences (in Neurology and in the Taub Institute) at the Columbia University Medical Center
sa2969@cumc.columbia.edu

