Columbia University
Irving Medical Center
Neurological Institute
710 West 168th Street, 3rd floor
(212) 305-1818
TaubCONNECT Research Perspective:
October 2024
2: Multi-Omic Analysis of Huntington's Disease Reveals a Compensatory Astrocyte State
3: Cytoplasmic Vacuolation and Ectopic Formation of Perineuronal Nets Are Characteristic Pathologies of Cytomegalic Neurons in Tuberous Sclerosis
4: Cognitive Polygenic Index Is Associated with Occupational Complexity Over and Above Brain Morphometry
Epigenetic and Genetic Risk of Alzheimer Disease from Autopsied Brains in two Ethnic Groups
 |  |  | ||
| Yiyi Ma, MD, PhD | Richard Mayeux, MD, MSc | Badri N. Vardarajan, PhD, MS |
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder multiple etiologies. We studied both genetic variants and epigenetic features that contribute to the risk of AD by focusing our study on genetic variants that affect CpG sites (we called them CpGrelated single nucleotide polymorphisms (CGS)). CpG dinucleotides are a key part of epigenetic mechanisms in mammalian somatic cells. They are the only site in these cells where DNA methylation can occur, and they also harbor mutation hotspots. CpG sites are found in high frequency in genomic regions known as CpG islands, which are short DNA sequences with a high concentration of CpG and CG compared to the rest of the genome. In humans, about 70% of promoters near a gene's transcription start site contain a CpG island. CGSes or variants that affect CpG sites act as the hub of both the genetic and epigenetic effects because they alter the sequence of CpG dinucleotides which are the target site of DNA methylation.
|
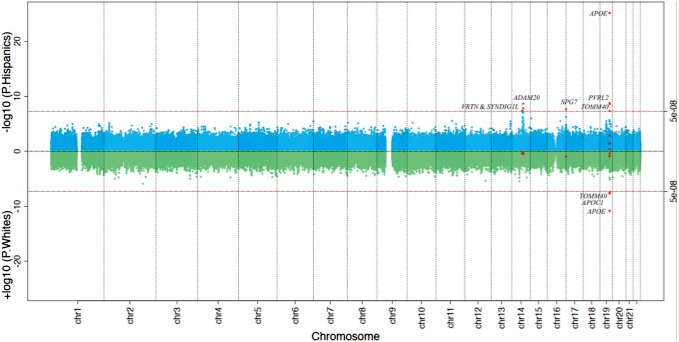
Figure 1. Sliding CGS window search across the genome for the risk loci of clinical diagnosis of Alzheimer disease in Hispanics and non-Hispanic Whites. The genome-wide sliding window results for the Hispanics (upper panel in blue) and the non-Hispanic Whites (NHW) (lower panel in green) are shown in the miami plot. Each dot represents one 1-Kb window, and X and Y axis shows its genomic coordinate and -log10 transformed P value. The two horizontal red lines show the Bonferroni-corrected genome-wide significance threshold (P≤ 5×10-8) and those CGS windows passing the genome-wide significance threshold in either Hispanics or NHW are shown in red dots.
|
In our recently published study in Acta Neuropathologica, we conducted a genome-wide, sliding-window-based association with AD, in 7,155 on Caribbean Hispanics (CH) CH and 1,283 Non-Hispanic Whites (NHW). Next, we evaluated the cis- and trans-effects of these associated CGSs on methylation and gene expression in the dorsolateral pre-frontal cortex in 179 CH brains. We identified six genetic loci in CH with CGS dosage associated with AD at genome-wide significance levels: ADAM20 (Score=55.19, P=4.06x10-8), the intergenic region between VRTN and SYNDIG1L (Score=-37.67, P=2.25x10-9), SPG7 (16q24.3) (Score=40.51, P=2.23x10-8), PVRL2 (Score=125.86, P=1.64x10-9), TOMM40 (Score=-18.58, P=4.61x10-8), and APOE (Score=75.12, P=7.26x10-26). CGSes in PVRL2 and APOE were also significant in NHW. Except for ADAM20, CGSes in all the other five loci were associated with CH brain methylation levels (mQTLs) and CGSes in SPG7, PVRL2, and APOE were also mQTLs in NHW. Except for SYNDIG1L (P=0.08), brain methylation levels in all the other five loci affected downstream mRNA expression in CH (P<0.05), and methylation at VRTN and TOMM40 were also associated with mRNA expression in NHW. Gene expression in these six loci were also regulated by CpG sites in genes that were enriched in the neuron projection and synapse (FDR<0.05). DNA methylation at all the six loci and SYNDIG1 and TOMM40 gene expression were significantly associated with Braak stage In summary, we identified six CpG associated genetic loci associated with AD in CH, harboring both genetic and epigenetic risks. However, their downstream effects on mRNA expression maybe ethnic specific and different from NHW.
Yiyi Ma, MD, PhD
Assistant Professor of Neurological Sciences (in Neurology and in the Gertrude H. Sergievsky Center)
ym2666@cumc.columbia.edu
Badri N. Vardarajan, PhD, MS
Assistant Professor of Neurological Science (in Neurology, the Sergievsky Center, and the Taub Institute)
bnv2103@cumc.columbia.edu
Multi-Omic Analysis of Huntington's Disease Reveals a Compensatory Astrocyte State
 |
 |
| Vilas Menon, PhD | Osama Al-Dalahmah, MD, PhD |
Huntington’s disease (HD) is an incurable neurodegenerative disease caused by an expansion of CAG repeats in the HTT gene locus, leading to neuronal loss and astrogliosis. Despite the presence of the mutation in all brain cells, neuronal loss follows a classic distribution, the hallmark of which is severe degeneration of the striatum compared to cortical regions. Even within the striatum, the caudate nucleus shows more severe degeneration when compared to the nucleus accumbens, despite the relative similarity of the cell types that inhabit both regions. In the current study, now published in Nature Communications, we explored the role of astrocyte heterogeneity in the cell-type specific and regional vulnerability to neurodegeneration in HD. We used a combination of transcriptomic, lipidomic, and neuropathologic approaches to determine the relationships between specific astrocyte states and prototypic neurodegeneration patterns in HD.
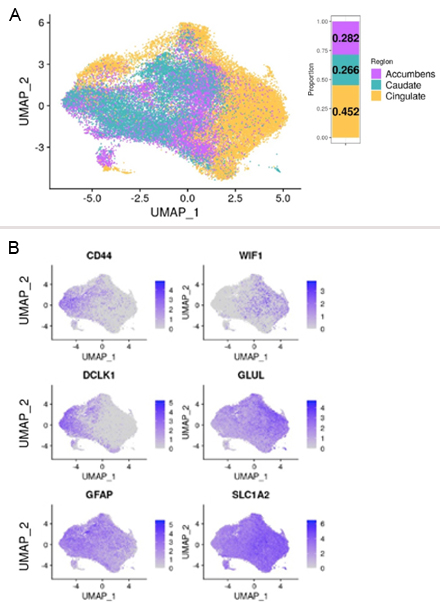
Figure 2. A) UMAP plot of astrocytes projected in isolation of other cell types, and color-coded by region. The bar plot on the top right shows the distribution of astrocytes between the three brain regions. B) Feature plots of normalized gene expression projected in the UMAP embeddings to highlight genes that differentiate fibrous-like (top) and protoplasmic astrocytes (bottom).
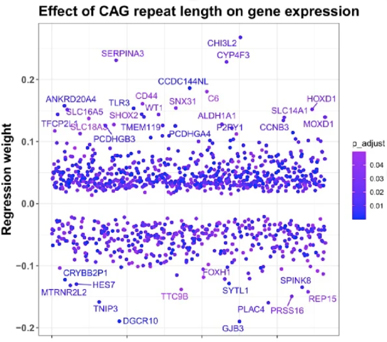
Combining bulk-level transcriptomics approaches with paired measurements of CAG repeat lengths allowed us to identify a set of genes that were increased as a function of CAG repeat length (Figure 1). These genes were enriched in glial and astrocytic genes and several pathways related to fatty acid metabolism – which is one of the primary functions of astrocytes. Indeed, lipidomic studies of cortical HD and control samples revealed several lipid species to be dysregulated in HD. Among the lipid species that increased in HD were long-chain polyunsaturated fatty acids, and we found them to be toxic to neurons in culture. Notably, astrocytes process polyunsaturated fatty acids, which further implicates astrocytes in HD pathology.
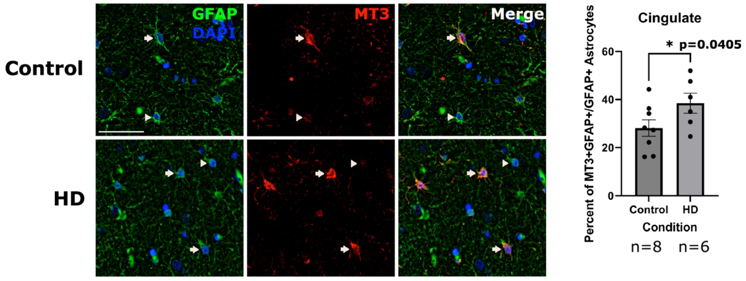
Motivated by these findings, we measured gene expression using single nucleus RNAseq from over 60 brain samples, including 20 HD and 10 controls across three brain regions: The cingulate cortex, the caudate nucleus, and the nucleus accumbens. We identified more than 45,000 astrocytes across the three brain regions (Figure 2A) and classified them as protoplasmic or CD44+ fibrous-like astrocytes (Figure 2B). HD caudate protoplasmic astrocytes showed higher enrichment of the gene signature correlated with CAG repeat length. Interestingly, we discovered that HD protoplasmic astrocytes in the cingulate cortex and nucleus accumbens, where neurodegeneration is less severe, showed high levels of metallothionein genes – which encode metal-sequestering anti-oxidative stress proteins. We confirmed these findings at the protein level in post-mortem human HD and control brain sections (Figure 3).
|
Figure 3. Immunofluorescent images of the caudate labeled for nuclei (DAPI-blue) and GFAP (green) to detect astrocytes (left panel), and MT3 (red-middle panel). A merge of the three channels is shown on the right. Arrows indicate DAPI, GFAP and MT3 positive cells (MT3 positive astrocytes) and arrowheads indicate MT3 negative astrocytes. Scale bar = 50 μm. Quantification of the percent of percent of astrocytes that were MT3 positive in the cingulate (Right). Unpaired one-tailed t-test with n = 8 for control and 6 for HD. Data are shown as mean ± SEM.
|
As we describe in our paper, astrocyte states were regionally heterogeneous. One state was characterized by elevated metallothionein protein expression, and was depleted from the caudate nucleus but enriched in less severely affected regions – the cingulate cortex and the nucleus accumbens. We sought to determine the impact of this astrocyte state on neuronal viability in collaboration with Dr. Andrew Yoo from Washington University in St. Louis, who developed a method to convert patient fibroblasts directly to striatal-type spiny projection neurons. We co-cultured astrocytes that upregulated one of the metallothionein proteins, MT3, or control astrocytes with HD patient-derived spiny striatal projection neurons. We found that compared to control astrocytes, neurons cocultured with MT3-astrocytes exhibited lower levels of markers of cell death, including Annexin V. We also confirmed the neuroprotective effect of MT3 astrocytes on neurons using another model of neurodegeneration in vitro. Thus, our findings indicate that the MT-high astrocyte state is compensatory or neuroprotective.
Altogether, our findings uncover heterogeneity of astrocyte states in the HD brain that correlates with the regional vulnerability to neurodegeneration. Further research is needed to causally connect astrocyte states to vulnerability to neurodegeneration. In addition to addressing this knowledge gap, we plan to explore the utility of compensatory astrocyte states as a therapeutic modality in HD, and in other neurodegenerative diseases.
Vilas Menon, PhD
Assistant Professor of Neurological Sciences (in Neurology and the Taub Institute)
vm2545@cumc.columbia.edu
Osama Al-Dalahmah, MD, PhD
Assistant Professor in Pathology and Cell Biology
oa2298@cumc.columbia.edu
 |  | |
| Alexander A Sosunov, MD,PhD | Guy M. McKhann II, MD
| |

|  | |
| Guomei Tang, PhD | James E.Goldman, MD, PhD |
Tuberous Sclerosis Complex (TSC) is a developmental disorder caused by mutations in the genes, TSC1 or TSC2, leading to a loss of function of the encoded proteins. These proteins normally inhibit the activation of a kinase, the mechanistic target of rapamycin complex 1 (mTORC1), and so the loss of these proteins will produce an abnormal activation of mTORC1. The disorder leads to the generation of collections of abnormal neurons and glia in the cortex, called “tubers”, as well as subventricular neoplasms, the so-called subependymal giant cell astrocytomas. Tubers are often the origins of seizures. In addition, many of the patients with TSC suffer from autistic spectrum disorders. One of the major cellular components of tubers are highly enlarged “cytomegalic” neurons, which develop from the pyramidal neurons of the isocortex.
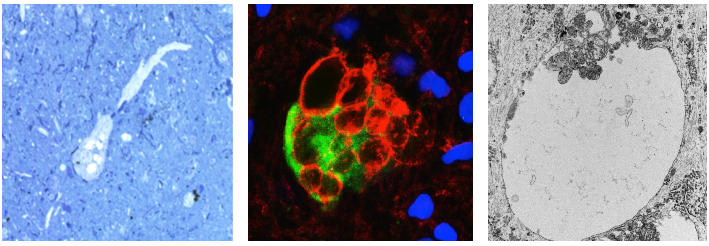
Our group at Columbia published a paper in 2022 in Cell Reports, showing that in a mouse model in which Tsc1 is inactivated in late embryonic radial glial cells, the cytomegalic neurons receive enhanced excitatory input and contribute to cortical hyperexcitability and seizures. We also noticed that many of these cytomegalic neurons contained cytoplasmic vacuoles following chronic seizures. In a study recently published in Journal of Neuropathology and Experimental Neurology, Alexandre Sosunov, Guy McKhann II, Guomei Tang, and I further explored these vacuoles in the mouse model and in human tubers. The vacuole membranes contain plasma membrane components, including KCC2, the chloride channel that maintains a low intracellular chloride concentration so that GABA will produce synaptic inhibition. Many of these neurons show little KCC2 on the plasma membrane, suggesting that the intracellular chloride concentration may be increased. Our study proposes that the vacuoles arise from the Golgi network and represent a neuron’s inability to transfer Golgi membranes to the plasma membrane.
|
Figure 1. At the left is a vacuolated neuron from a human tuber. In the middle, a tuber neuron is filled with
vacuoles immunostained with an antibody against KCC2 (red), which outlines the vacuoles. At the right is the ultrastructure of a vacuole, single membrane bound, with invaginations of cytoplasm protruding into it.
|
Another feature of these abnormal neurons is that they are surrounded by peri-neuronal nets, a special organization of extracellular matrix, which is a normal feature of inhibitory neurons, but rarely present in excitatory cortical neurons. These nets may change the synaptic input of the neurons. We examined human tubers from autopsy and surgical excisions and found similar cytoplasmic vacuoles and peri-neuronal nets in the cytomegalic neurons. These pathological states of tuber neurons contribute to cortical hyperexcitability and seizure progression of individuals with TSC. How the abnormal activation of mTORC1 produces these abnormalities is not yet clear.
James E.Goldman, MD, PhD
Professor of Pathology and Cell Biology (in Psychiatry)
jeg5@cumc.columbia.edu
 |  | |
| Angeliki Tsapanou, PhD | Yaakov Stern, PhD |
The relationship between occupation and cognitive skills has been well researched; however, there is limited research on whether genetically predicted cognitive scores influence occupational choices. Given the significant role that occupation plays in an individual's life and well-being, identifying factors that may predispose cognitively healthy adults to certain occupations is crucial. In the current study, recently published in Behavior Genetics, we explored the association between the Cognitive Polygenic Index (PGI) and occupational choice in cognitively healthy adults aged 20-80 years.
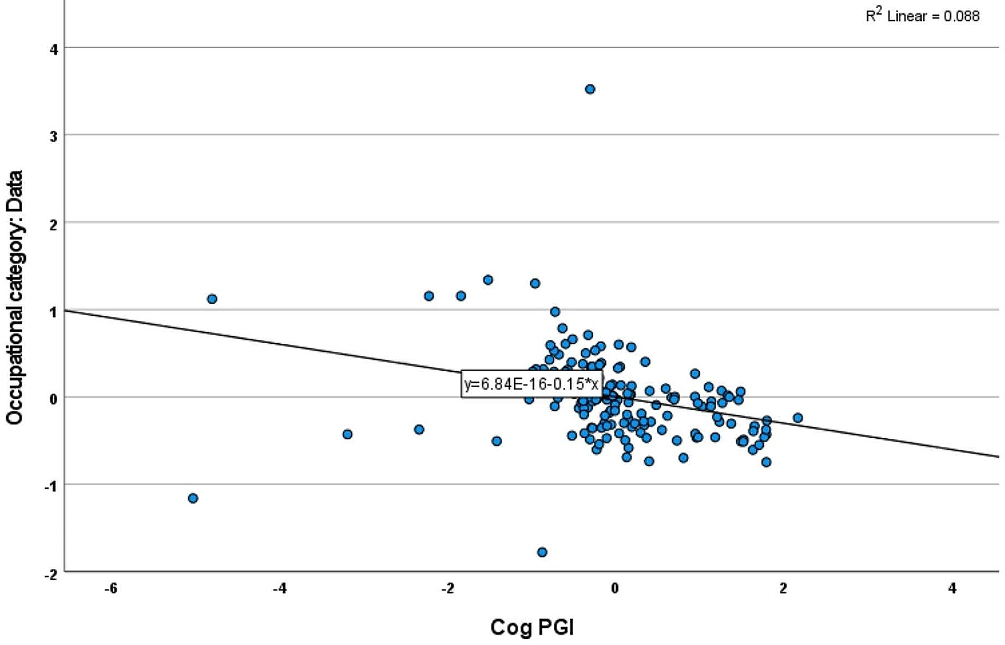
Participants were drawn from two studies: the Reference Ability Neural Network (RANN) and the Cognitive Reserve (CR) study. Occupational complexity, measured across Data, People, and Things, was assessed using O*NET descriptors. Using a previously created Cognitive PGI and linear regression models, we analyzed 168 participants of white ethnicity, aged 20-80 years, with an average of 16 years of education. After controlling for factors such as age, sex, education, and principal components, we found a significant association between Cognitive PGI and the complexity of Data-related occupations. Higher Cognitive PGI was linked to higher Data complexity, while no significant associations were found for People or Things. Even after adjusting for brain morphometry measures like total cortical thickness and gray matter volume, the association with Data complexity remained significant (B=-0.059, SE: 0.029, p=0.045).
Our findings suggest that individuals with higher Cognitive PGI are more likely to pursue occupations with greater Data complexity, accounting for their brain morphometry. This suggests that genetic variation associated with cognition may predispose individuals for occupational choices.
Angeliki Tsapanou, PhD
Associate Research Scientist in the Gertrude H. Sergievsky Center
at2859@cumc.columbia.edu
Yaakov Stern, PhD
Professor of Neuropsychology (in Neurology, in Psychiatry, in the Gertrude H. Sergievsky Center, and in the Taub Institute)
ys11@cumc.columbia.edu