Columbia University
Irving Medical Center
Neurological Institute
710 West 168th Street, 3rd floor
(212) 305-1818
Featured Research
In the Lab:
Vilas Menon, PhD

Vilas Menon, PhD
The brain is an organ of immense complexity, comprising multiple classes of cells with distinct molecular signatures. In brain disease, the interactions among these different cell types are dysregulated, leading to changes in signaling, processing, and cell health that ultimately result in severe cognitive and motor impairment.
In order to uncover the main modes of dysregulation in diseases of aging, our group generates and analyzes large-scale molecular data from brain tissue and model systems, with a special focus on Alzheimer’s Disease (AD). In conjunction with other groups at the Taub Institute, we use single cell and tissue-level molecular techniques—single-nucleus RNA-seq, bulk RNA-seq, and proteomics, among others—to generate profiles of brain tissue and individual brain cells. To tame the subsequent deluge of data from thousands of molecular species in tens of thousands of samples, we develop novel analytical, computational, and machine learning approaches to identify putative cell types and potential interactions among these types. These techniques allow us to prioritize candidate cell signatures and genes that are likely associated with pathology and clinical symptoms in neurodegeneration.
From our analysis of post-mortem tissue obtained from donors with and without clinical diagnoses of AD, we have identified multiple subsets of cells that show changes associated with neurodegeneration. Together with fellow Taub and Center for Translational and Computational Neuroimmunology (CTCN) faculty members Marta Olah, Elizabeth Bradshaw, Wassim Elyaman, and Philip De Jager, we have identified changes in the composition of microglia, the resident immune cells of the brain, in donors with diagnoses of AD. We have also extracted putative interactions between subsets of neurons and astrocytes (Figure 1), highlighting candidate genes and cell signatures that may ultimately be targetable and have therapeutic applications in AD.
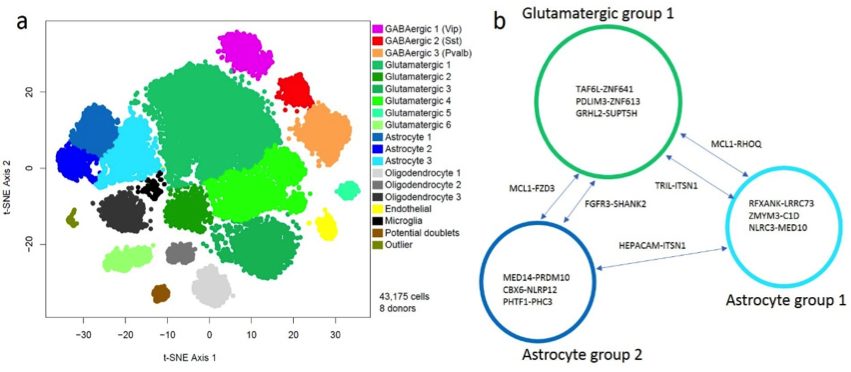 Figure 1. A visualization (left) of tens of thousands of individual nuclei from post-mortem human brain samples. Each point represents a nucleus, and they are arranged such that nuclei with similar molecular profiles are close together in space. Each color represents a group of nuclei with a distinct molecular signature. The right panel shows candidate gene-gene interactions among three groups of cells, extracted from the same data set, that are associated with Alzheimer’s Disease – this leads to the hypotheses that dysregulation of these interactions may lead to pathological and clinical manifestations of Alzheimer’s Disease.
|
Given the broad applicability of our data generation techniques and computational approaches to analyze large-scale data sets, our group also has active collaborations with multiple faculty members studying all aspects of neurodegeneration, including Serge Przedborski (for Parkinson’s Disease), Gunnar Hargus (for frontotemporal dementia), and Neil Shneider (for ALS).
Vilas Menon, PhD
Assistant Professor of Neurological Sciences (in Neurology and the Taub Institute for Research on Alzheimer Disease and the Aging Brain)
Center for Translational and Computational Neuroimmunology (CTCN)
vm2545@cumc.columbia.edu

