Columbia University
Irving Medical Center
Neurological Institute
710 West 168th Street, 3rd floor
(212) 305-1818
TaubCONNECT Research Perspective:
June 2023
2: Dietary Flavanols Restore Hippocampal-Dependent Memory in Older Adults with Lower Diet Quality and Lower Habitual Flavanol Consumption
3: Survey of Neuroanatomic Sampling and Staining Procedures in Alzheimer Disease Research Center Brain Banks
 |  | |
| Lawrence S. Honig, MD, PhD | Richard Mayeux, MD, MSc |
While cerebrospinal fluid (CSF) and blood biomarkers can uncover biological signs of Alzheimer's disease (AD), their application is limited in under-resourced settings and among diverse ethnic groups. New research from Taub faculty members Drs. Lawrence Honig, Richard Mayeux, and colleagues, aimed to assess validated plasma biomarkers for AD among adults of Caribbean Hispanic ethnicity. In this cross-sectional study, 746 Caribbean Hispanic adults from the Dominican Republic and New York underwent thorough clinical evaluations and blood sampling, with a subset agreeing to spinal fluid sampling. The main findings related to the association of blood biomarkers amyloid-β 1-42 (Aβ42), amyloid-β 1-40 (Aβ40), total tau (T-tau), phosphorylated tau181 (P-tau181), glial fibrillary acidic protein (GFAP), and neurofilament light chain (NfL) with an AD diagnosis. These biomarkers allow for the evaluation of amyloid (A), neurofibrillary degeneration (T), and neurodegeneration (N) components of AD. Exposure factors included age, sex, education level, country of residence, number of apolipoprotein-ε4 (APOE-ε4) alleles, serum creatinine level, blood urea nitrogen level, and body mass index. The average age of participants was 71 years (64.3% of whom were women), with 20.6% meeting the clinical criteria for AD.
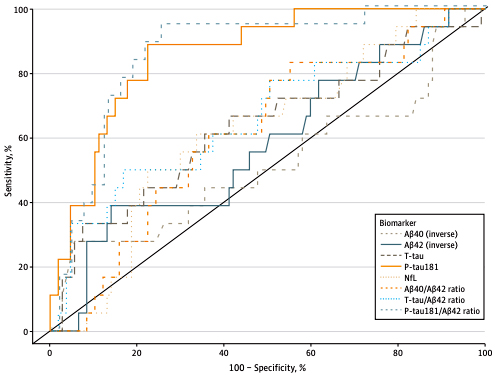
Figure. Receiver Operator Curve Analyses of Plasma Biomarker Performance in Classifying Cerebrospinal Fluid (CSF)–Supported Diagnosis of Alzheimer Disease Performance of plasma biomarkers amyloid-β 1-40 (Aβ40) (inverse), amyloid-β 1-42 (Aβ42) (inverse), total tau (T-tau), neurofilament light chain (NfL), phosphorylated tau181 (P-tau181), and ratios of Aβ40/Aβ42, T-tau/Aβ42, and P-tau181/Aβ42 in classifying CSF biomarker (P-tau181/Aβ42)–supported diagnosis of biological Alzheimer disease.
As recently reported in JAMA Network Open and featured on the CUIMC Newsroom, utilizing plasma cut-off points established with 127 individuals with both CSF and plasma data, Honig et al. compared the clinical diagnoses derived from comprehensive cognitive and functional assessment with plasma biomarkers. Defining the outcome as biological AD and considering plasma biomarkers as evidence of AD pathology, they found that 54.6% of clinically diagnosed AD individuals had biological AD based on plasma P-tau181, and 41.1% based on plasma P-tau181/Aβ42. However, in individuals without dementia, 22.7% had P-tau181 levels above the cut-off point and 17.7% had P-tau181/Aβ42 above the cut-off point, indicative of a biological AD despite being without current symptoms. Those individuals with AD biomarkers, namely elevated P-tau181 or P-tau181/Aβ42, regardless of clinical diagnosis, were more often carriers of the APOE-ε4 allele, consistent with AD; they also had elevated GFAP levels consistent with neurodegeneration
A significant number of individuals with clinical dementia lacked plasma AD biomarkers and did not meet the A/T/N classification; this group might include those with non-AD dementias, early AD with low cognitive reserve, or mixed dementia. But for clinical AD, molecular plasma biomarkers improve diagnostic specificity. Among individuals without dementia, a proportion had biomarker-positive status, likely indicating the presence of incipient AD in these individuals – and the likelihood that they might therefore develop clinical symptoms over time. Plasma biomarkers, like PET or CSF, may identify premorbid AD among otherwise healthy individuals without dementia, thereby increasing the sensitivity of the neuropsychological and functional assessment in this study.
Overall, this study significantly contributes to the existing literature, advocating for the use of plasma-based biomarkers in observational and clinical studies as a cost-effective, practical method to improve diagnostic specificity, particularly in low-resource settings where options for functional brain imaging and CSF sampling are restricted. Furthermore, plasma biomarkers may offer a means to identify individuals in the early, asymptomatic stages of AD, and allow for treatment trials before the onset of cognitive or behavioral symptoms.
Lawrence S. Honig, MD, PhD
Professor of Neurology (in The Taub Institute, The Sergievsky Center)
lh456@cumc.columbia.edu
Richard Mayeux, MD, MSc
Gertrude H. Sergievsky Professor of Neurology, Psychiatry and Epidemiology (in the Sergievsky Center and Taub Institute)
rpm2@cumc.columbia.edu
 |  | |
| Adam M. Brickman, PhD | Scott A. Small, MD |
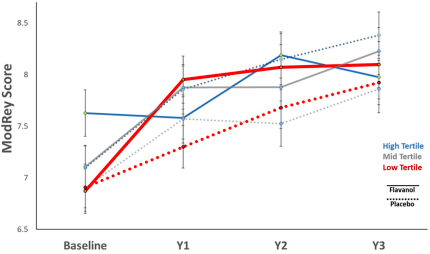
Certain dietary components may be crucial not only during neurodevelopment, but potentially also as the brain ages. Previous research by Taub faculty Drs. Adam Brickman, Scott Small, and colleagues demonstrated an association between flavanols, dietary elements found in select fruits and vegetables, and improved cognition in older adults. More recently, Drs. Brickman, Small, and colleagues conducted a 3-month clinical trial across a range of flavanol intake amounts that showed flavanols to be differentially linked to hippocampal-dependent cognition, with the most consistent flavanol-induced memory improvement observed in participants in the lowest tertile of habitual diet quality.
In their latest work, Brickman and Small collaborated with researchers at Brigham and Women’s Hospital to design a web-based ancillary study—COSMOS-Web—within the larger COSMOS (COcoa Supplement and Multivitamin Outcomes Study) trial, to further assess the effects of flavanols on cognition and cognitive aging in the hippocampus. Here, 3,562 elderly participants were randomly assigned to a three-year regimen of either a cocoa extract (containing 500mg of cocoa flavanols daily) or a placebo. As recently reported in PNAS and featured in the CUIMC Newsroom, using the alternate Healthy Eating Index for all participants and a urine-based biomarker to measure flavanol intake in a subset of participants (n = 1,361), they found that regular consumption of flavanols and a quality diet were positively and specifically correlated with memory dependent on the hippocampus.
While the initial primary goal of memory improvement for all participants at the one-year mark was not statistically significant, the flavanol regimen improved memory among participants with lower quality diets or lower regular flavanol consumption. Increases in the flavanol biomarker over the trial were associated with improved memory. Overall, their findings provide biomarker-based evidence that dietary consumption of flavanols can be etiologically linked to age-related memory decline.
Adam M. Brickman, PhD
Professor of Neuropsychology in Neurology (in The Taub Institute, The Sergievsky Center)
amb2139@cumc.columbia.edu
Scott A. Small, MD
Boris and Rose Katz Professor of Neurology (in The Taub Institute, The Sergievsky Center, Radiology and in Psychiatry)
sas68@cumc.columbia.edu

Andrew F. Teich, MD, PhD
As NIA expands funding for Alzheimer’s disease research, federally funded Alzheimer’s disease research centers (ADRCs) have become ever more central to the research infrastructure. This applies both to established centers such as Columbia’s ADRC, as well as new centers that have been granted during the funding expansion. The neuropathology core is a mandated component of these centers and serves to diagnose and bank post-mortem brain and spinal cord from patients with Alzheimer’s disease and related dementias. In Vizcarra et al., Dr. Andrew Teich and colleagues performed a survey of best practices across ADRC neuropathology cores, motivated by several factors. First, as new centers have been granted, neuropathology core leaders new to the ADRC system have requested data on what other centers are doing as they set up their own facilities. Dr. Teich serves on the national ADRC neuropathology core steering committee, and this is an area where the committee can assist through issuing this survey. In parallel, digital pathology has become increasingly prominent in ADRC neuropathology cores, which raises the exciting prospect of cores sharing slide images, allowing for larger histology-based studies. One major hurdle to implementing this vision is understanding how to put together a standard “slide set” for imaging purposes that is compatible with what is already being collected across centers.
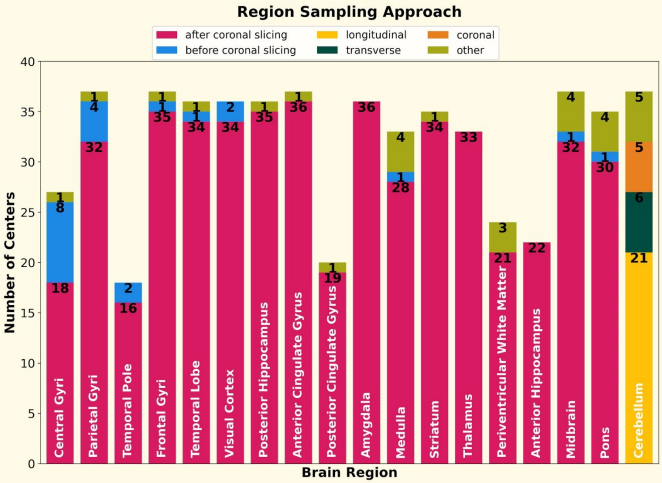
Figure 3. For 18 out of the 19 regions (olfactory bulb excluded), the number of centers that sample the region via different sectioning approaches is shown using stacked bar plots. For 17 regions, the survey asked if the centers sampled the region after or before coronal slicing. For the cerebellum region, the options were: longitudinal, transversal, and coronal slicing. The numbers show the value of the stacked bar. The label in each bar is the brain region.
This survey was designed to address both of these needs and was issued jointly by the ADRC neuropathology steering committee (chaired by Dr. Teich), and the digital pathology working group (chaired by Dr. Brittany Dugger). The results were presented at the fall 2022 ADRC meeting and published in Free Neuropathology. Response rate was high, with 38/40 centers responding. Centers consistently sampled several regions important to ADRD pathology, include the frontal gyri, visual cortex, midbrain, posterior hippocampus, and striatum (37/38 centers). Although immunohistochemical staining was similar in many areas, there was a surprising diversity in the number of antibodies used, with 12 different antibodies for beta-amyloid across centers, and significant variability in whether antibodies were against phospho- or non-phospho- epitopes for alpha-synuclein and TDP-43. Section thickness also varied from center to center, and although several areas were commonly sampled, the anatomic landmarks used to take these sections showed more variability, suggesting that the resulting slides are unlikely to be perfectly aligned across centers, even for the “same” sampled area.
In summary, this survey provides data to new centers and also serves as a starting point to discuss a common slide set that centers could opt into for purposes of a national digital pathology archive. Work towards this latter goal is ongoing by both the ADRC steering committee and the digital pathology working group, and the results of this survey will facilitate future funding requests.
Andrew F. Teich, MD, PhD
Associate Professor of Pathology and Cell Biology (in Neurology)
aft25@cumc.columbia.edu