Columbia University
Irving Medical Center
Neurological Institute
710 West 168th Street, 3rd floor
(212) 305-1818
TaubCONNECT Research Perspective:
June 2024
2: The Broken Alzheimer's Disease Genome
3: Personality Traits and Cognitive Reserve—High Openness Benefits Cognition in the Presence of Age-Related Brain Changes
Updated Safety Results From Phase 3 Lecanemab Study in Early Alzheimer's Disease

Lawrence S. Honig, MD, PhD
The development of FDA-approved disease modifying therapies for Alzheimer's disease (AD) has been a major achievement in neurotherapeutics over the past few years. AD, which is a disease that affects cognition and memory, is caused by excessive beta-amyloid protein in the brain, which is deposited in the form of neuritic plaques, and is associated with the development of neurofibrillary degeneration and neurodegeneration including synaptic and ultimately neuronal cell losses. The clinical effects of AD, with disorientation, amnesia, language and visuospatial problems, and behavioral and functional impairments, places an enormous burden on the 7 million Americans, and others throughout the world, who are affected, and on their families, as well on healthcare systems. The development of therapies that slow the progression of disease has changed neurological care for persons with dementia. The most-used therapy is lecanemab, which is a monoclonal antibody that is administered intravenously and binds with high affinity to soluble beta-amyloid in the form of protofibrils, which are more toxic to neurons than monomeric beta-amyloid or insoluble polymeric forms of beta-amyloid. The drug lecanemab (also known as BAN-2401) is approved for use in the US, China, and Japan, after phase 2 and phase 3 studies proved that it decreased cerebral beta-amyloid levels, resulting in the marked clearance of beta-amyloid deposits known as senile neuritic plaques, and showed clear clinical benefits.
In the late 2010’s, an 18-month, phase 2, dose-finding study of lecanemab, using a Bayesian adaptive design was conducted in 856 participants with mild cognitive impairment (MCI) due to AD and mild dementia due to AD dementia (collectively termed “early AD”). This study showed removal of cerebral amyloid as measured by PET scans, favorable changes in a variety of fluid biomarkers, and evidence of dose-dependent clinical benefits. In the phase 2 study, as in prior studies of anti-amyloid antibodies, there were also side effects, most notably the dose-dependent development of brain edema and brain hemorrhages, called amyloid-related imaging abnormalities (ARIA). Following the phase 2 study, a phase 3, randomized, double-blind, placebo-controlled trial (called Clarity AD) with an open-label extension (OLE) was performed. This study confirmed lecanemab clinical efficacy, with slowing of cognitive and functional decline by a variety of neuropsychological and functional outcome measures, to the extent of 26% to 37% over the 18-month trial period, and resulted in the FDA-approval of the drug, and subsequent approval by regulatory authorities in Japan and China. While overall the drug was well tolerated in these trials, lecanemab treatment was again accompanied by not only some manageable infusion reactions (with greater incidence than in placebo), but also with ARIA-E (ARIA with edema or effusion) and ARIA-H (ARIA marked by microhemorrhages, superficial siderosis, or very rarely macrohemorrhages greater than 1 cm in diameter). ARIA-E and ARIA-H appear to be related to cerebral amyloid angiopathy (CAA), which in turn is significantly related to APOE-ε4 allele dosage.
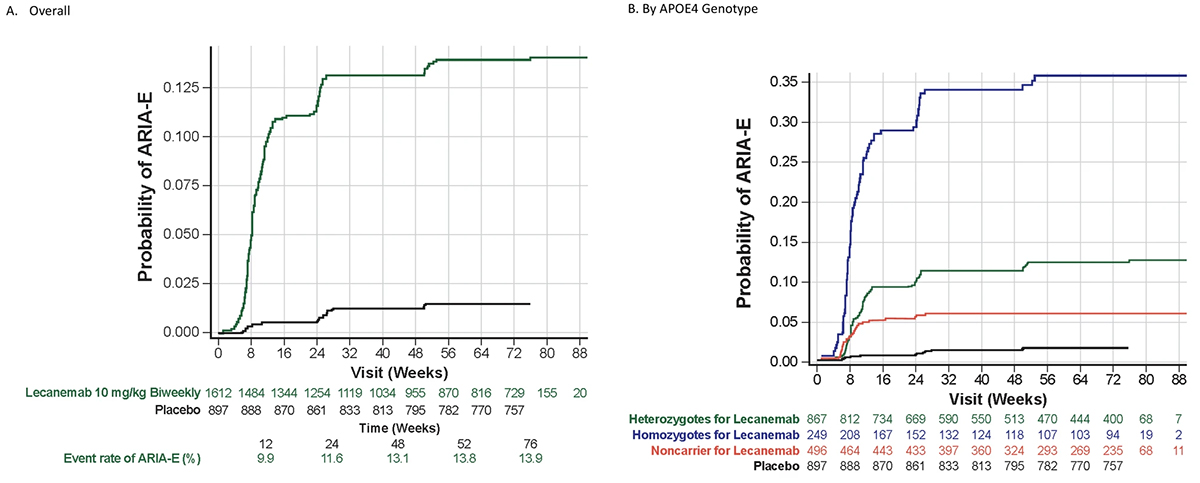
Now published in Alzheimer’s Research & Therapy, this publication presents detailed safety information from the double-blind Core phase and the OLE of the phase 3 Clarity AD study in early Alzheimer's disease. The data shows the serious adverse events and deaths which occurred during the study, and also more detailed and updated data on the cerebral side effects of ARIA-E and ARIA-H, complementing previously published trial data from the phase 2 and phase 3 Core studies. The phase 3 trial included 1795 participants in the Core phase and 1612 in the Core + OLE phases, with lecanemab showing good tolerability. During the Core phase there was no increase in deaths with lecanemab (0.7% deaths on lecanemab versus 0.8% deaths on placebo), but some increase in serious adverse events (14% on lecanemab vs 11% on placebo). Overall, frequent adverse events more common in the lecanemab group included infusion reactions (24.5%), ARIA-H (16.0%), and ARIA-E (13.6%). ARIA-E and ARIA-H events were predominantly mild-to-moderate in radiological severity; only about one in four ARIA-E events were symptomatic. Major findings are that ARIA-E occurs mostly in the first 3-6 months of therapy, and is much more common in ApoE ε4 homozygotes (33%), than in heterozygotes (11%), or non-carriers (5%); it is usually asymptomatic, and typically resolves over 90 days. ARIA-H microhemorrhages that are “isolated”, which is to say not occurring in the context of ARIA-E, are not clearly increased in incidence in those who received lecanemab treatment (8.7%) compared to those who received placebo (7.7%). Lecanemab treatment did seem to be associated with increase in isolated ARIA-H superficial siderosis, and likely in the rare macrohemorrhages that occurred in less than 0.5% of lecanemab-treated patients. ARIA-H is markedly related to ApoE genotype, with a monotonic increase in microhemorrhages over time, regardless of drug treatment, but occurring much more so in APOE e4 homozygotes, than heterozygotes or non-carriers.
The safety information on lecanemab is important for discussing risks and benefits in patients considering anti-amyloid therapy with lecanemab, and for understanding the FDA-mandated monitoring procedures, including three brain MRI studies in the first seven months of treatment with this drug. As increased numbers of patients receive anti-amyloid therapies, it is most important to have a thorough understanding of the potential risks, and the procedures to mitigate these risks through monitoring by healthcare providers, patients, and caregivers ensuring optimal patient management and care.
Lawrence S. Honig, MD, PhD
Professor of Neurology (in the Gertrude H. Sergievsky Center and the Taub Institute)
lh456@cumc.columbia.edu
The Broken Alzheimer's Disease Genome
 |  |  | ||
| Cláudio Gouveia Roque, PhD | Hemali Phatnani, PhD | Ulrich Hengst, PhD |
Genomics and related big data disciplines have revolutionized our understanding of Alzheimer’s disease. However, no single genomic modality, no matter how rich in detail, provides a complete picture of the disease on its own. From genetics to proteomics, all biological layers contribute interconnected pieces of evidence.
In "The Broken Alzheimer’s Disease Genome," we explore this complementarity and offer a comprehensive overview of how genomic research has advanced our thinking of late-onset Alzheimer’s beyond the classical pathology hallmarks. Our review, now published in Cell Genomics, facilitates an integrated discussion of recent genetic, epigenetic, transcriptomic, and proteomic findings. This broad framing is used deliberately: by thinking holistically rather than narrowly about specific processes, we strived to promote dialogue across subfields. Our emphasis on the synergies among data modalities results in a unique and rich perspective that substantially expands upon prior systematization efforts. We further discuss clinical applications, such as risk prediction tools and biomarkers, and outline how genomics is shaping drug development.
Cláudio Gouveia Roque, PhD
New York Genome Center
croque@nygenome.org
Hemali Phatnani, PhD
Assistant Professor of Neurological Sciences (in Neurology)
hp2286@cumc.columbia.edu
Ulrich Hengst, PhD
Professor of Pathology and Cell Biology (in the Taub Insitute)
uh2112@cumc.columbia.edu
 |  | |
| Annabell Coors, PhD | Yaakov Stern, PhD |
It is known that some people are less susceptible to age-related pathological changes in the brain and therefore perform better on cognitive tasks than would be expected given their brain (health) status. These individuals are said to have higher cognitive reserve (CR). It is important to gain a deeper understanding of what sets them apart. We can use this knowledge to learn how to age healthier and to identify individuals who are at increased risk of cognitive decline and need targeted interventions. We hypothesized that personality influences leisure behavior and may therefore be linked to the level of CR.
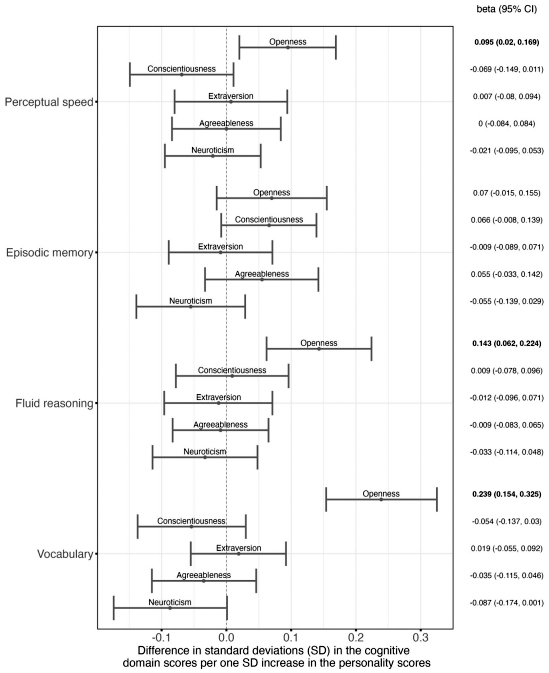
Figure 2. Personality and Cognitive Domain Scores. This forest plot shows how much performance in different cognitive domains (see y-axis) differs per one SD increase in each of the BIG 5 personality dimensions. Associations were adjusted for the cognitive domain-specific brain status variable, age, and sex. Each dot represents the standardized estimate for the association, while the lines around it show the 95%-confidence interval belonging to this estimate. The corresponding values are shown in the column on the right side with significant associations printed in bold. Notably, all dots on the right side of the dotted vertical line indicate that higher values in the respective personality dimension are associated with better cognitive performance in the specific domain, while all dots on the left side indicate that higher values in the respective personality dimension were associated with lower cognitive performance.
Thus, we measured the personality traits openness, conscientiousness, extraversion, agreeableness, and neuroticism with a questionnaire and examined whether they underlie CR throughout adulthood. Leveraging both cross-sectional (N=399) and longitudinal (N=273) data, we assessed the cognitive abilities of healthy individuals between 19 to 80 years old across different cognitive domains (perceptual speed, memory, fluid reasoning, vocabulary). To quantify brain status, we used MRI-derived cortical thickness and volume variables of brain regions associated with cognitive performance.
As recently reported in the journal Neurobiology of Aging, our findings suggest that individuals with higher levels of openness tend to have superior fluid reasoning abilities and vocabulary, even after adjusting for brain status, age, and sex. Moreover, a worse brain status at baseline assessment was associated with decline in perceptual speed only in individuals with lower levels of openness. In individuals with high openness, brain status at baseline was not related to change in perceptual speed. This suggests that high openness - a personality trait characterized by creativity, imagination, and interests in new things - underlies higher CR. Contrary to our expectations, high neuroticism (low emotional stability) did not underlie lower CR. We believe that some previous studies found this association because their study samples included individuals who already had or were on the verge of developing Alzheimer’s disease. Alzheimer’s disease could be a common underlying cause of both high neuroticism and low CR, rather than high neuroticism and low CR being directly related.
The key message of our research project is that it is beneficial to be and remain open to experience, as high openness helps to counteract the negative effects of age-related brain pathology on cognition. Importantly, a person’s personality is not set in stone and can change, especially in response to major life changes such as marriage, divorce, or unemployment. Unfortunately, many people show a decline in openness in older age, which may be due to reduced exposure to new and challenging contexts. For them, engagement in volunteer work or training in new skills and competencies could foster high levels of openness and protect against cognitive decline.
Annabell Coors, PhD
Postdoctoral Research Scientist in the Taub Institute
ac5256@cumc.columbia.edu
Yaakov Stern, PhD
Florence Irving Professor of Neuropsychology (in Neurology, Psychiatry, the Gertrude H. Sergievsky Center and the Taub Institute)
ys11@cumc.columbia.edu