Columbia University
Irving Medical Center
Neurological Institute
710 West 168th Street, 3rd floor
(212) 305-1818
TaubCONNECT Research Perspective:
March 2023
2: Healthy Lifestyle Behaviors and Biological Aging in the US National Health and Nutrition Examination Surveys 1999-2018
 |  | |
| Cláudio Gouveia Roque, PhD | Ulrich Hengst, PhD |
Ever since the emergence of genome-wide profiling methodologies, gene expression changes have been one of the most researched molecular features of Alzheimer’s disease. These system-level studies are highly informative and can be used to inform unbiasedly on specific aspects of disease pathogenesis. A potential drawback is the complexity of these readouts, as it is not uncommon for thousands of genes to be highlighted in comparisons between affected and healthy individuals. An open question in the field has been whether these changes are causative agents of disease progression or a mere bystander response to the pathophysiological process accompanying Alzheimer’s. By initially focusing on a single transcriptional pathway regulated by β-amyloid, an early trigger of Alzheimer’s decline, our latest work provides strong evidence for the importance of gene expression alterations in the pathological cascade of this neurodegenerative disorder.
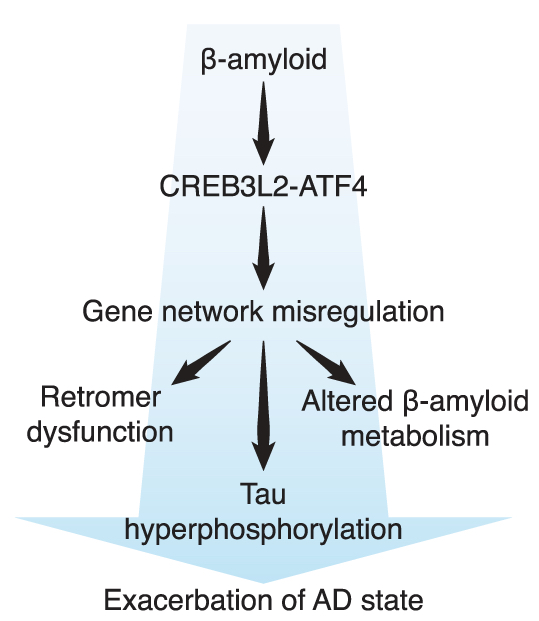
Figure 6. CREB3L2-ATF4 interacts with β-amyloid and tau neuropathologies. (G) The findings support a model whereby CREB3L2-ATF4 promotes AD-linked gene expression changes and contributes to characteristic features of AD pathophysiology, including retromer dysfunction, altered β-amyloid metabolism, and tau hyperphosphorylation.
The findings, published recently in Science Advances, were driven by a well-known but typically disregarded aspect of transcription factor biology: their dependency on dimerization for binding to DNA. Building on previous research by our group, we first identified CREB3L2 as a new binding partner of ATF4, a central effector of cellular responses to stress. Interestingly, while CREB3L2-ATF4 dimers can be found in neurons under normal conditions, the interaction between these two transcription factors is substantially potentiated in neurons challenged by β-amyloid. Subsequent efforts focused on elucidating the transcriptional response downstream of CREB3L2-ATF4, which included the development of a novel, broadly accessible genomic technique for studying transcription factor dimers.
Crossing this dataset with brain gene expression profiles of Alzheimer’s disease sufferers led to the identification of several relevant pathways linked to pathogenesis, including retromer dysfunction and tau regulation. Further experiments demonstrated that the transcriptional response activated by CREB3L2-ATF4 is sufficient to trigger tau hyperphosphorylation in neurons. In other words, the work now online provides direct support for a transcriptional link between the two hallmark lesions associated with Alzheimer’s disease, β-amyloid and tau. Finally, by characterizing CREB3L2-ATF4 in detail, our lab hopes to use this knowledge to guide the identification of a promising candidate drug, dovitinib, which can normalize gene expression downstream of β-amyloid. Unlike available anti-amyloid therapies, dovitinib is predicted to act downstream of β-amyloid and may one day be used as a complementary approach to mitigate the harmful effects mediated by this disease insult.
Cláudio Gouveia Roque, PhD
Associate Research Scientis in the Taub Institute
cag2230@cumc.columbia.edu
Ulrich Hengst, PhD
Associate Professor of Pathology and Cell Biology & Taub Insitute
uh2112@cumc.columbia.edu
 |  |  | ||
| Aline Thomas, PhD | Daniel W. Belsky, PhD | Yian Gu, MD, MS, PhD |
Healthy lifestyle behaviors, including balanced diet and regular physical activity, are associated with reduced risk for multiple chronic diseases and longer lifespan. Diet and exercise are also associated with preservation of metabolic, immunologic, and physical functioning with aging. These observations suggest the hypothesis that healthy lifestyle behaviors may slow the pace of biological aging.
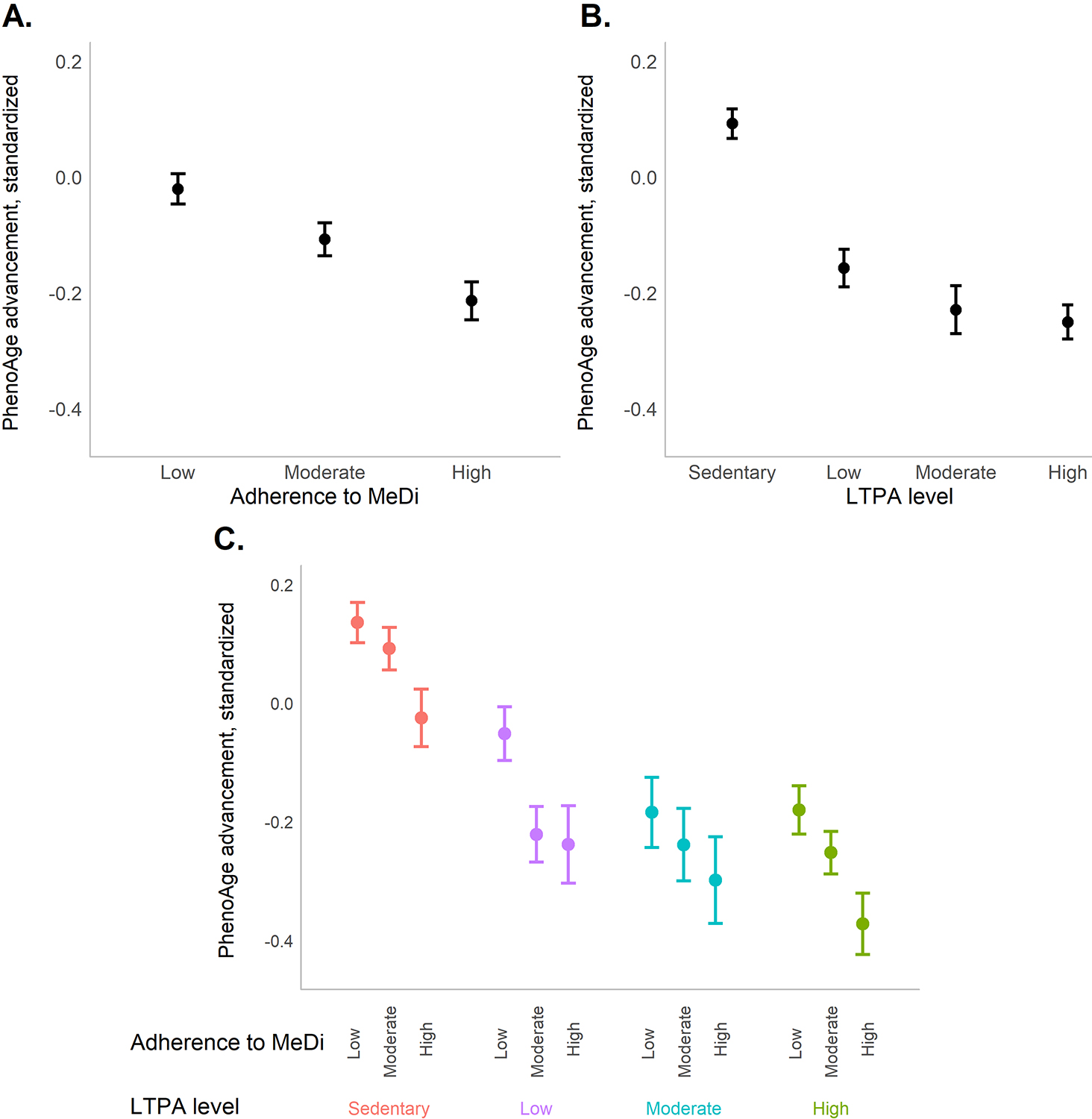
Figure. Mean standardized PhenoAge advancement by MeDi and LTPA categories, NHANES 1999-2018 (n = 42,625)
Notes: LTPA = leisure-time physical activity; MeDi = Mediterranean diet. Means and 95% confidence intervals of the standardized PhenoAge advancement weighted estimates accounting for sampling design, by categories of adherence to MeDi (Panel A; low [0-3], moderate [4-5], and high [6-9]), LTPA levels (Panel B; sedentary [0 MET min/week], low [1-500 MET min/week], moderate [500-1000 MET min/week], and high [›1000 MET min/week]), and the interaction of MeDi and LTAP categories (Panel C)
In our recent study published in The Journals of Gerontology: Series A, we tested this hypothesis using data from the National Health and Nutrition Examination Surveys (NHANES; 1999-2018). In 42,625 participants aged 20-84 years, we calculated adherence to a Mediterranean diet (MeDi) and level of leisure time physical activity (LTPA). Additionally, we measured biological aging by applying the PhenoAge algorithm, developed as a predictor of mortality and morbidity using data from NAHNES III (1988-1994), to clinical chemistries measured from a blood draw at the time of the survey. We tested the associations of MeDi and LTPA measures with biological aging, explored synergies between these health behaviors, and tested heterogeneity in their associations across strata of age, sex, and body mass index. We found that higher MeDi adherence was associated with PhenoAge advancement across categories of LTPA, and a higher LTPA level was associated with lower PhenoAge advancement across the MeDi categories, with no evidence of interaction. In addition, across NHANES waves and for all individuals (younger and older, males and females, lean and participants with obesity), a healthier diet and a higher level of physical activity were consistently associated with younger biological age.
Overall, these consistent associations encourage the promotion of diet and physical activity for overall healthy aging to the general population.
Aline Thomas, PhD
Postdoctoral Research Scientist in the Taub Institute
at3702@cumc.columbia.edu
Daniel W. Belsky, PhD
Associate Professor of Epidemiology in the Robert N. Butler Columbia Aging Center
db3275@cumc.columbia.edu
Yian Gu, MD, MS, PhD
Associate Professor of Neurological Sciences in Neurology, Epidemiology in the Gertrude H. Sergievsky Center and the Taub Institute
yg2121@cumc.columbia.edu

