Columbia University
Irving Medical Center
Neurological Institute
710 West 168th Street, 3rd floor
(212) 305-1818
TaubCONNECT Research Perspective:
February 2025
2: Sleep Genetics and Cognitive Changes over Time: The Moderating Effect of Age and the Role of Brain
3: Emerging Roles for Tubulin PTMs in Neuronal Function and Neurodegenerative Disease

Wassim Elyaman, PhD
Established in 2023 by Dr. Wassim Elyaman, the T Cells in the Brain (TCB) symposium has rapidly emerged as a premier platform for advancing neuroimmune research. Following the success of its first event in January 2024, TCB expanded into a two-day symposium in January 2025, convening leading experts from industry, basic and translational science, technology, genetics, and clinical research. Partnering with five national institutions and backed by the NIA and the Alzheimer’s Association, TCB has fostered critical discussions on the role of T cells in neurological diseases, where identifying effective therapeutic targets remains a significant hurdle.
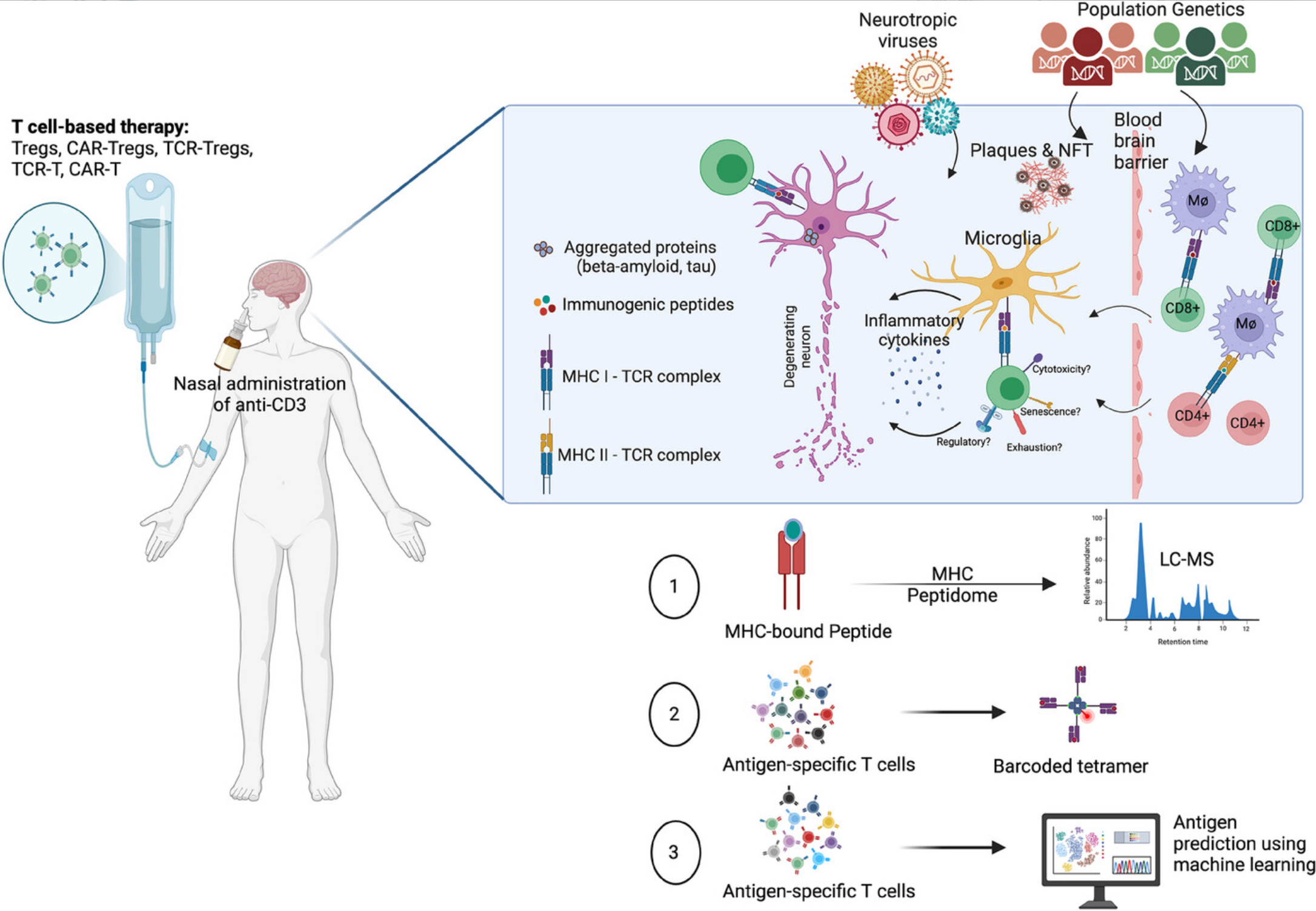
Figure 1. Schematic illustration of research topics presented at the T Cells in the Brain 2024 symposium, highlighting the discovery of antigens presented on MHC class I and II molecules in the context of neurodegenerative diseases, and the identification of clonally expanded T cells, their phenotypes, and antigen specificity using single-cell RNA/TCR sequencing (scRNA/TCR-seq) and TetTCR-seqHD. See full caption.
The symposium aimed to address this challenge by integrating recent scientific breakthroughs with innovative approaches to T cell-based therapies. Discussions covered key areas such as antigen-specific immune responses, TCR repertoire dynamics, and microglia-T cell interactions, alongside state-of-the-art technologies including immunopeptidome profiling, high-dimensional tetramer-based screening, and spatial mapping of TCR clonotypes in the human brain. Insights from TCB 2024, recently published in Alzheimer’s & Dementia, underscore the symposium’s impact on neuroimmune research. Notable advancements featured include regulatory T cell-based therapies for ALS, spearheaded by Dr. Neil Shneider, and the VALAD trial for Alzheimer’s, led by Dr. Davangere Devanand.
Wassim Elyaman, PhD
Assistant Professor of Neurological Sciences (in Neurology and the Taub Institute)
we2152@cumc.columbia.edu
Sleep Genetics and Cognitive Changes over Time: The Moderating Effect of Age and the Role of Brain
 |  | |
| Angeliki Tsapanou, PhD | Yaakov Stern, PhD |
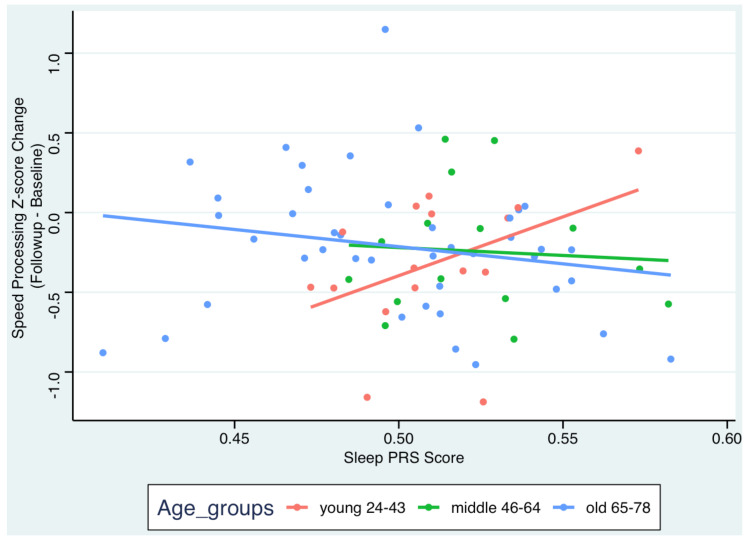
In our previous studies, we have noted that several measures of sleep play a crucial role in cognitive performance and cognitive changes in aging. In the current study, recently published in Genes, we investigated the role of sleep duration genetics (Sleep polygenic index, Sleep PGI) in cognitive changes over a five-year follow-up and the moderating effect of age. A total of 96 participants were included, aged 24 to 78 years old, recruited from the Reference Abilities Neural Network and the Cognitive Reserve studies. The cognitive domains of memory, executive function, attention/speed of processing and language were examined.
In the entire sample, with or without adjusting for age, sex, education, genetic principal components and structural brain imaging measures (mean cortical thickness, total gray matter volume), Sleep PGI was not associated with cognitive change over time for any of the cognitive domains. However, we found that age moderated the association between Sleep PGI and changes in the cognitive domain of speed of processing, after adjusting for the above covariates. The results indicated that a longer Sleep PGI was associated with a better speed of processing performance over time in younger but not older adults.
Genetic variants associated with sleep duration significantly influence performance in speed of processing, with age playing a critical moderating role, over and above brain morphometry. A genetic predisposition for longer sleep duration can work as a protective factor against decline in the speed of processing in young adults. With age, other factors such as environment may play more important role in cognition.
Angeliki Tsapanou, PhD
Associate Research Scientist in the Sergievsky Center
at2859@cumc.columbia.edu
Yaakov Stern, PhD
Florence Irving Professor of Neuropsychology (in Neurology, Psychiatry, the Sergievsky Center, and the Taub Institute)
ys11@cumc.columbia.edu
 |  | |
| JiaJie Teoh, PhD | Francesca Bartolini, PhD |
Emerging Roles for Tubulin PTMs in Neuronal Function and Neurodegenerative Disease
Tubulin and microtubules play a vital role not only in neurodevelopment but also in the progression of neurodegenerative diseases. In this minireview, published in Current Opinion in Neurobiology, we explore how specific tubulin post-translational modifications (PTMs) impact both postsynaptic and presynaptic functions in the adult central nervous system and contribute to disease pathology. Beyond their established roles in organelle transport, these modifications also appear to govern various synaptic activities, such as dendritic spine dynamics and neurotransmitter release.
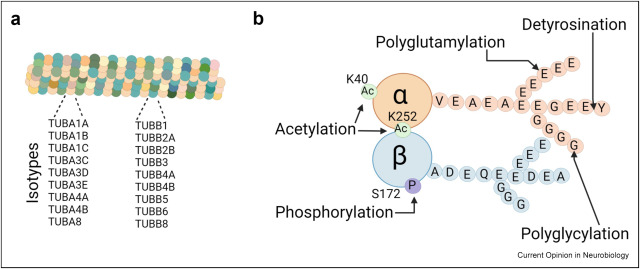
Figure 1. Microtubules are built from α/β tubulin heterodimers, combining 9 α-tubulin and 9 β-tubulin isotypes in mammals, each with distinctive C-terminal tails. (b) Tubulin heterodimer composed of α and β tubulins. The combinatorial nature of posttranslational modifications of tubulin such as detyrosination, acetylation, polyglutamylation, polyglycylation and phosphorylation create a "tubulin code" that regulates several microtubule-dependent neuronal functions.
These observations raise the possibility that targeting the enzymes responsible for adding or removing these tubulin PTMs, the tubulin “writers” and “erasers,” could help restore neural circuit integrity in neurodegenerative conditions. Despite these advances, our grasp of the full spectrum of their functions remains in the early stages, leaving several intriguing questions unanswered.
Francesca Bartolini, PhD
Associate Professor of Pathology and Cell Biology
fb2131@cumc.columbia.edu