Columbia University
Irving Medical Center
Neurological Institute
710 West 168th Street, 3rd floor
(212) 305-1818
TaubCONNECT Research Perspective:
February 2024
2: Whole Genome-Wide Sequence Analysis of Long-Lived Families (Long-Life Family Study) Identifies MTUS2 Gene Associated with Late-Onset Alzheimer's Disease
3: In Vivo Tau is Associated with Change in Memory and Processing Speed, but not Reasoning, in Cognitively Unimpaired Older Adults
4: The Effects of Insufficient Sleep and Adequate Sleep on Cognitive Function in Healthy Adults
Glucocorticoid Stress Hormones Stimulate Vesicle-Free Tau Secretion and Spreading in the Brain

Clarissa Waites, PhD
Exposure to stressful life events and elevated levels of glucocorticoids (GCs), the primary hormones associated with stress, are recognized as significant risk factors for Alzheimer’s disease (AD). A pivotal pathological link between stress and AD appears to be the protein Tau. Stress and elevated GC levels induce Tau pathologies similar to those observed in AD, such as Tau hyperphosphorylation and oligomerization. Moreover, experimental reductions in Tau have been found to offer protection against the neurotoxic effects and cognitive deficits induced by amyloid beta and stress in animals, underscoring the pivotal role of Tau in the neurodegenerative processes linked to AD and prolonged stress.
A hallmark of AD-related Tau pathology is its distinctive pattern of spread among regions of the brain that are anatomically connected, specifically from the entorhinal cortex to the hippocampus, and then to the prefrontal cortex. This pattern of Tau dissemination is closely linked to the degree of cognitive dysfunction observed in AD patients and is considered a critical factor in the disease‚Äôs advancement. For more than a decade, research in this area has primarily concentrated on the role of vesicles in the distribution of Tau, yet the vast majority of Tau secreted by neurons (‚ÄČ~‚ÄČ90%) is vesicle-free. This non-vesicular secretion pathway and its impact on the spread of Tau pathology are not well understood. Additionally, the exact mechanisms by which stress and GCs may facilitate Tau‚Äôs pathological spread remain to be clarified.
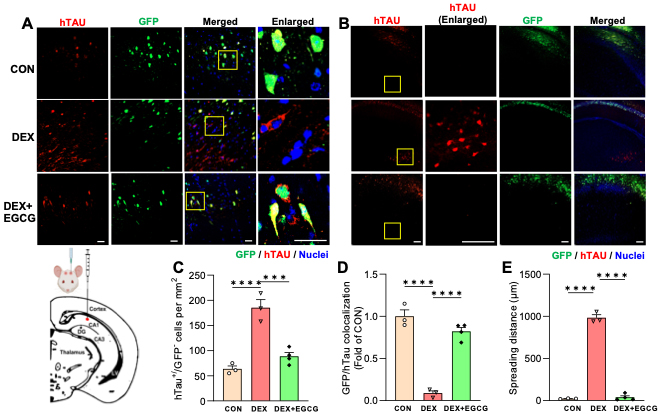
Figure. Glucocorticoids induce Tau spreading in the brain, which is blocked by an inhibitor of Tau oligomerization. A-B) Images of human Tau (hTau) spreading in the mouse hippocampus. GFP labels AAV-infected neurons, hTau immunostaining labels neurons with hTau, hTau+/GFP- neurons indicate Tau spreading. The synthetic glucocorticoid dexamethasone (DEX) induces hTau spreading within (A) and beyond (B) hippocampal area CA1, where AAV was injected (see schematic diagram). EGCG, an inhibitor of Tau oligomerization and type 1 unconventional protein secretion, prevents DEX-induced spreading. Yellow boxes indicate enlarged regions. Size bars, 25 um. C-E) Quantification of Tau spreading based on hTau+/GFP- neurons per mm2 (C), GFP/hTau colocalization (D), and maximum spreading distance from the injection site (E). ***P<0.001, ****P<0.0001, one-way ANOVA with multiple comparisons.
In the current study, recently published in Cell Death & Disease, we explored the impact of GCs on Tau secretion and its spread in mouse hippocampal neurons and ex vivo brain slices. We discovered that GCs trigger the release of primarily vesicle-free Tau, a process that is dependent on neural activity and glycogen synthase kinase 3ő≤ (GSK3ő≤), occurring via type 1 unconventional protein secretion (UPS). Furthermore, we found that GC treatment enhances Tau spread across the hippocampus, an effect that can be mitigated by blocking Tau aggregation and type 1 UPS using the catechin epigallocatechin gallate. These results suggest that increased levels of GCs facilitate the propagation of Tau, offering insights into how stress and GCs may accelerate cognitive decline in AD.
Clarissa Waites, PhD
Associate Professor of Pathology and Cell Biology and Neuroscience
cw2622@cumc.columbia.edu

Sandra Barral, PhD
The Long-Life Family Study (LLFS) is an international collaborative study designed to examine the genetic and non-genetic factors associated with exceptional longevity. Previous studies examining the health of LLFS participants have shown that this cohort appears to be healthier than random cohorts of the same age/sex, including lower rates of dementia and memory decline. This exceptionally healthy cohort provides a unique opportunity to investigate the genetic contributions to Late Onset Alzheimer’s Disease (LOAD).
Our study examined the association between whole genome sequence variants and LOAD in 3,475 LLFS members. We further investigated whether the observed LOAD associated LLFS variants generalize to cohorts characterized by different risk of dementia: high risk populations (familial LOAD and Down Syndrome), Alzheimer‚Äôs disease referral-base cohort, and population-based cohorts. As recently reported in Alzheimer‚Äôs & Dementia, we identified several genetic variants within the MTUS2 gene significantly associated with LOAD. The association was also observed in the six independent cohorts considered and become significantly stronger within high Aő≤42/40 ratio compared to lower amyloid ratio.
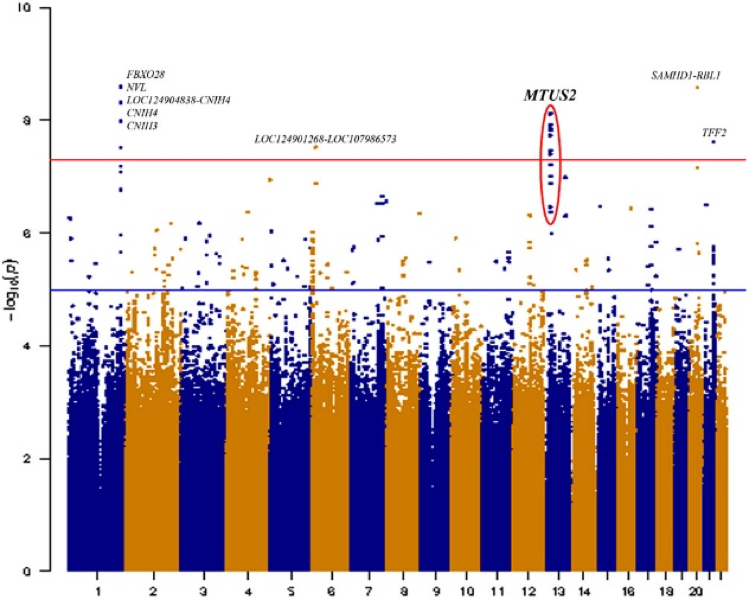
Figure 1. Manhattan plot of Seq-GWAS results in LLFS cohort. The X-axis represents the genomic position for each of the SNPs analyzed; Y-axis represents the -log10 transformed p-values. The red line indicates the genome-wide significance threshold (p < 5 √ó 10‚ąí8). GWAS, genome-wide association studies; LLFS, Long-Life Family Study; SNP, single nucleotide polymorphism.
MTUS2 is a plus end tracking protein implicated in the development and function of the nervous system. It is expressed in brain during development and adulthood. RNA-seq analysis of more than 1,100 individuals’ post-mortem brains from the Accelerating Medicines Partnership for Alzheimer’s disease (AMP-AD) consortium demonstrated significant change in MTUS2 gene expression when comparing LOAD cases and cognitively healthy samples. Our results support MTUS2 as a novel LOAD risk locus. Future functional validation analyses will be needed to further investigate the role of MTUS2 in LOAD pathology.
Sandra Barral, PhD
Associate Professor of Neurogenetics (in Neurology, the Taub Institute, and the Gertrude H. Sergievsky Center) at CUMC
smb2174@cumc.columbia.edu
 |  | |
| Sharon Sanz Simon, PhD | Yaakov Stern, PhD |
In vivo neuroimaging biomarkers of tau and amyloid-ő≤ pathology have become essential tools in research into normal cognitive aging and preclinical Alzheimer‚Äôs disease (AD), as deposition of both proteins begins several years before clinical symptoms emerge. Despite the well-accepted involvement of amyloid-ő≤ and tau pathology in AD, questions remain regarding the role of these proteins in age-related cognitive decline. For instance, previous studies found normal aging is associated with the deposition of tau pathology in the medial temporal lobe (MTL) and neocortex even in the absence of or in the context of low amyloid-ő≤ pathology. This condition has been termed primary age-related tauopathy and there is still debate as to whether this is part of the AD spectrum or is part of ‚Äúnormal‚ÄĚ aging. Previous work reported that tau pathology in transentorhinal regions precedes amyloid-ő≤ deposition and tau PET studies indicate a close relationship between patterns of early tau deposition and cognitive impairment.
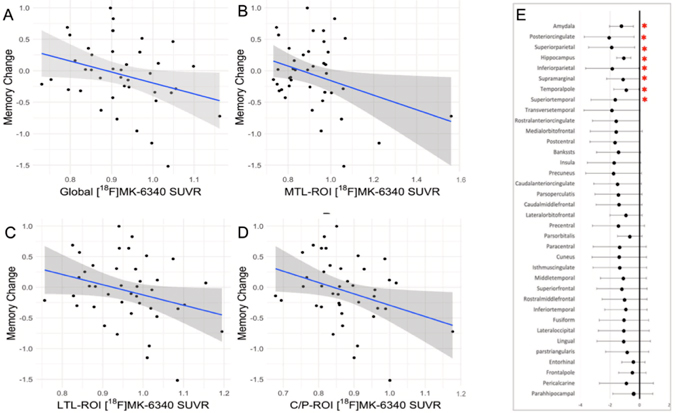
Figure 4. Scatterplot diagrams illustrating the relationship between tau PET uptake [ 18F-MK-6240] and memory change across brain regions. (A‚ÄďD) The associations considering regional tau uptake (meta-ROIs). (E) A forest plot representing the unstandardized betas and 95% interval confidence for each linear regression considering 36 brain areas. The results highlighted (*) are not corrected for multiple comparisons. All regressions accounted for age at follow-up, sex/gender, education, and baseline memory performance. Abbreviations: C/P, cingulate/parietal lobe; LTL, lateral temporal lobe; MTL, medial temporal lobe; ROI, region of interest; SUVR, standardized uptake value ratio.
Tau accumulation has been associated with longitudinal cognitive decline, typically in episodic memory. Due to the initial tau accumulation in the MTL, memory has been the domain typically investigated; however, few studies suggest tau accumulation to be associated with a decline in global cognition, executive functions, and speed processing. Therefore, the impact of early tau accumulation on cognitive trajectories beyond memory in cognitively unimpaired older adults still needs to be further characterized and understood, particularly with second-generation tau tracers. For instance, the tracer 18F-MK-6240 is a PET ligand for imaging neurofibrillary tangles in vivo that has shown favorable imaging characteristics and spatial distributions consistent with the neuropathological staging of neurofibrillary tangles in AD in preliminary studies. Studies evaluating 18F-MK-6240 in humans have indicated high affinity to neurofibrillary tangles in AD, minimal off-target binding in the brain, and the presence of extra-axial signals in some cases. In addition, the tracer 18F-MK-6240 was shown to predict cognitive decline in cognitively unimpaired older adults.
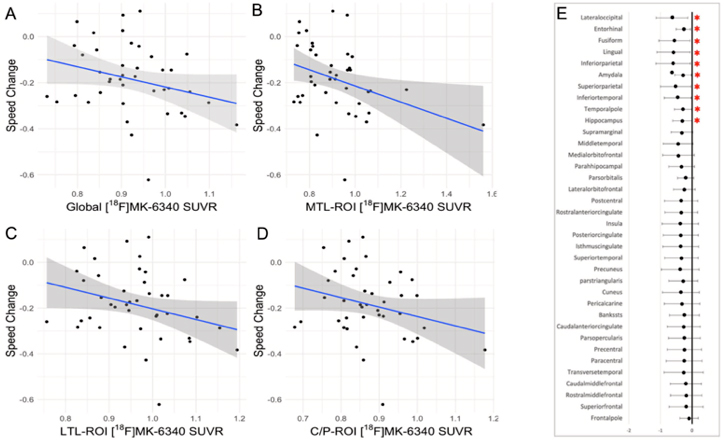
Figure 5. Scatterplot diagrams illustrating the relationship between tau PET [ 18F-MK-6240] and processing speed change across brain regions. (A‚ÄďD) The associations considering regional tau uptakes (meta-ROIs). (E) A forest plot representing the unstandardized betas and 95% interval confidence for each linear regression considering different 36 brain areas. The results highlighted (*) are not corrected for multiple comparisons. All regressions accounted for age at follow-up, sex/ gender, education, and baseline memory performance. Abbreviations: C/P, cingulate/parietal lobe; LTL, lateral temporal lobe; MTL, medial temporal lobe; ROI, region of interest; SUVR, standardized uptake value ratio.
Using data from the ongoing, longitudinal Reference Ability Neural Network Study, we evaluated the association of late-life tau deposition with retrospective cognitive change considering cognitive domains well established to decline with aging: episodic memory, speed processing, and reasoning. For tau quantification, a set of regions of interest (ROIs) was selected a priori: (1) total-ROI comprising selected areas, (2) medial temporal lobe-ROI, (3) lateral temporal lobe-ROI and (4) cingulate/parietal lobe-ROI. Using a sample of 41 cognitively healthy older adults, we found that higher levels of 18F-MK-6240 in brain regions linked to Alzheimer's disease‚ÄĒnamely, the medial temporal lobe, cingulate, and parietal regions‚ÄĒwere associated with faster declines in episodic memory and processing speed, but not in reasoning ability. This association persisted even when adjusting for demographic variables (like age, sex, and education), baseline cognitive function, and amyloid-ő≤ levels. As recently reported in Neurobiology of Aging, our findings highlight the role of pathological tau in areas susceptible to early buildup in driving cognitive changes seen in Alzheimer's disease, even in older adults without cognitive impairments and who have low amyloid-ő≤ levels. Despite the small sample size of the present study, our findings suggest that memory and processing speed declines typically seen in normal aging may be partly due to tau accumulation, emphasizing the importance of exploring long-term relationships between tau buildup and cognitive abilities beyond memory.
Sharon Sanz Simon, PhD
Associate Research Scientist (in the Taub Institute and the Gertrude H. Sergievsky Center)
sss2278@cumc.columbia.edu
Yaakov Stern, PhD
Florence Irving Professor of Neuropsychology (in Neurology, Psychiatry, the Gertrude H. Sergievsky Center, and the Taub Institute)
ys11@cumc.columbia.edu
The Effects of Insufficient Sleep and Adequate Sleep on Cognitive Function in Healthy Adults
 |  |  | ||
| Molly Zimmerman, PhD | Adam Brickman, PhD | Marie-Pierre St-Onge, PhD, CCSH, FAHA |
Sleep is essential for maintaining overall health and plays a pivotal role in cognitive processes vital for day-to-day functioning and overall quality of life, such as attention, memory, language, and executive functioning. Despite the focus on the negative cognitive effects of sleep deprivation, there has been little investigation into the impact of sleep consistency or the continuous achievement of sufficient sleep. Conducting such studies could broaden our knowledge of overall sleep health and potentially highlight a crucial target for treatment aimed at enhancing well-being.
In the current study, led by Dr. Molly Zimmerman, Professor of Psychology at Fordham University, in collaboration with Dr. Marie-Pierre St-Onge, Director of the Center for Excellence for Sleep & Circadian Research at Columbia, we assessed the cognitive effects of both insufficient and adequate sleep in 65 healthy adults, using a randomized, experimental approach. Our study also considered the potential influence of practice effects, which are improvements in test performance due to familiarity with the tasks rather than genuine cognitive gains. Differentiating between changes in cognition due to sleep quality and those due to repeated exposure to tests is crucial for accurate interpretation of the impact of sleep on cognitive function. We hypothesized that periods of insufficient sleep would lead to poorer cognitive performance compared to periods of adequate sleep, beyond the improvements attributed to task familiarity.
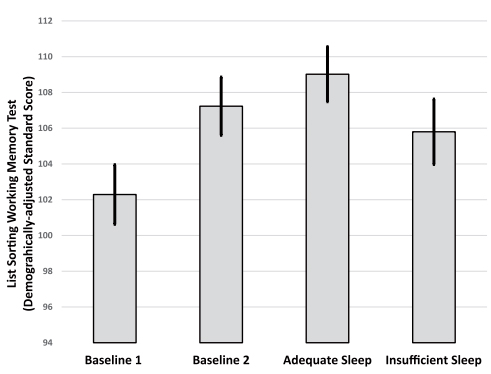
Figure 2. Differences in performance on the List Sorting Working Memory test from the NIH Toolbox across the four study visits. The improved score from the baseline of the first condition (B1) to the baseline of the second condition (B2) indicates a practice effect. Following the adequate sleep condition, participants showed greater improvement than what would be expected with practice. However, following the insufficient sleep condition, participants did not improve as much as would be expected with practice; there were no statistical differences between B1 and performance following the insufficient sleep condition. Values plotted are means (SE) derived from the repeated measures analysis of variance models.
As recently reported in Sleep Health, we found that maintaining adequate sleep consistently led to improvements in cognitive function, specifically working memory and response inhibition, surpassing the improvements that typically come from practice alone. Conversely, when individuals experienced consistently insufficient sleep, their ability to improve on cognitive function tests through practice was diminished compared to their performance with adequate sleep. These results contribute to our understanding of how various aspects of sleep quality and duration (including both the lack of sufficient sleep and the continuous maintenance of adequate sleep patterns) affect cognitive abilities. The findings indicate that ensuring a stable, consistent sleep schedule of at least 7 hours per night can enhance working memory and response inhibition in healthy adults.
Adam Brickman, PhD
Professor of Neuropsychology (in Neurology, the Taub Institute, and the Gertrude H. Sergievsky Center)
amb2139@cumc.columbia.edu

