Columbia University
Irving Medical Center
Neurological Institute
710 West 168th Street, 3rd floor
(212) 305-1818
TaubCONNECT Research Perspective:
February 2023
2: Genuine Selective Caspase-2 Inhibition with new Irreversible Small Peptidomimetics
3: Costs During the Last Five Years of Life for Patients with Clinical and Pathological Confirmed Diagnosis of Lewy Body Dementia and Alzheimer's Disease
 Francesca Bartolini, PhD
Francesca Bartolini, PhDMicroglia Reactivity Entails Microtubule Remodeling from Acentrosomal to Centrosomal Arrays
Microglia cells are a specialized cell type that acts as a “sentinel” in our brain and plays critical roles in neuroinflammatory and neurodegenerative disease. Activating signaling triggers a change in microglia morphology that reflects a highly activated state associated with phagocytosis and proinflammatory function. The intracellular mechanisms driving microglia transition from the homeostatic to a reactive state are however poorly understood. This is partially due to challenges in imaging purified microglia without perturbing their homeostatic or reactive state.
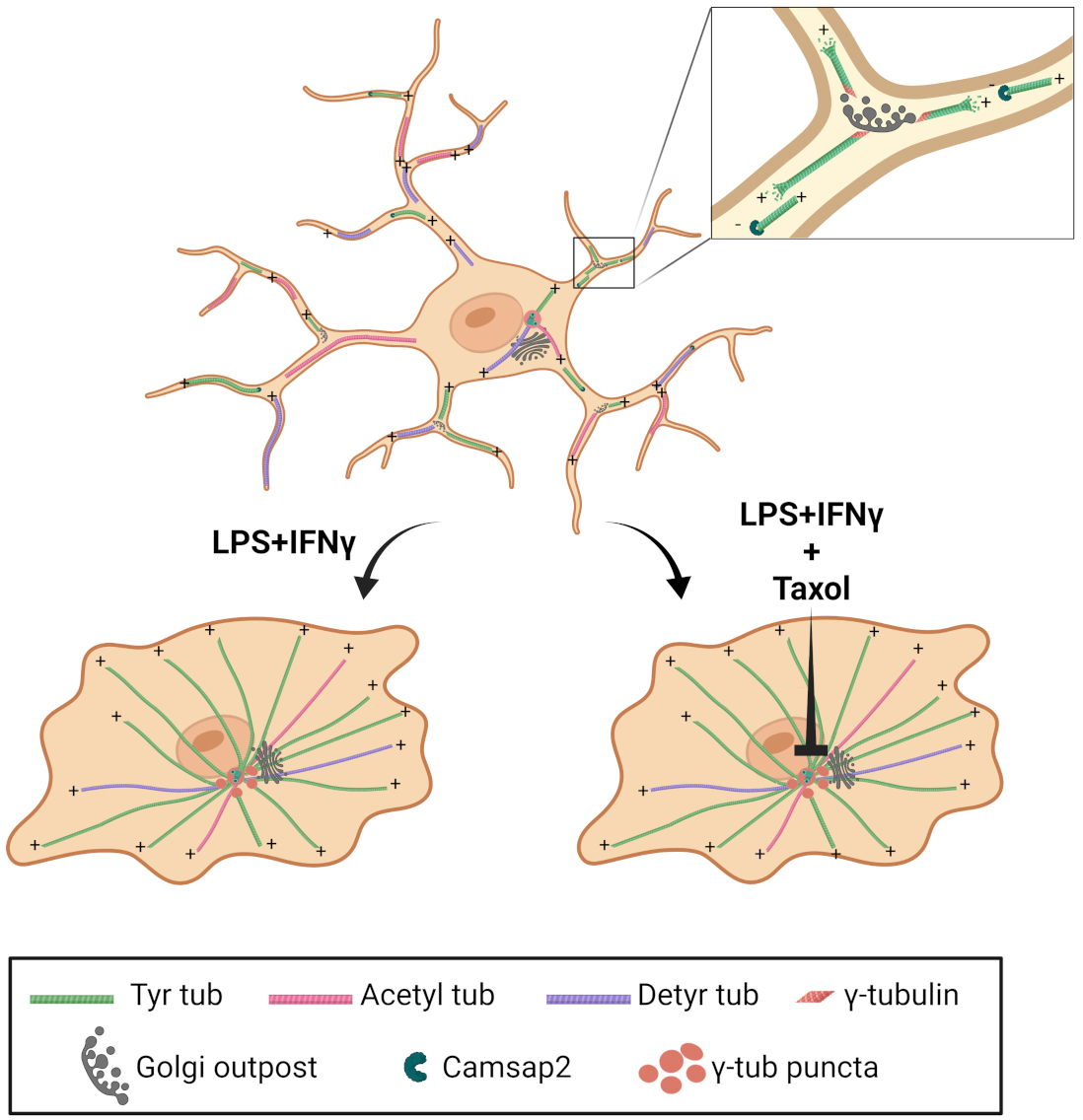
Graphical Abstract. Microglia cell transition between homeostatic and reactive states is characterized by a dramatic reorganization of the microtubule cytoskeleton from a Golgi outpost-nucleated acentrosomal array to a pericentrosomal radial array to enable proper cytokine release.
In this manuscript, through in vitro phenotyping and in vivo validation, the Bartolini laboratory, together with collaborators from “Sapienza” University and the Institute of Technology in Italy, reports the break-through discovery that reactive microglia undergo a dramatic reorganization of their microtubule cytoskeleton, the most abundant cytoskeleton in the brain. As recently published in Cell Reports, we found that reactive microglia engage a unique example of microtubule transition from an array of parallel microtubules nucleated at Golgi-outposts to a radial array in which all the microtubules are anchored to a pericentrosomal region. Our findings suggest that this reorganization is critical for supporting the microglia “patrolling” and proinflammatory function, providing a novel target for reducing microglia reactivity in inflammatory and neurodegenerative disease. Given the role for spinal microglia in the remission and recurrence of neuropathic pain, this study may also pave the way to determine the contribution of microglial microtubule dysfunction in the etiology of neuropathic pain caused by chemotherapeutic drugs, most of which target the microtubule cytoskeleton.
These spearheading observations will set the ground to visualize microtubule remodeling as a dynamic marker of microglia activation, and to examine the impact of microtubule targeting drugs on microglia reactivity in neurodegenerative disease and microglia-mediated regulation of synaptic function.
Francesca Bartolini, PhD
Associate Professor of Pathology and Cell Biology at CUIMC
fb2131@cumc.columbia.edu
Genuine Selective Caspase-2 Inhibition with new Irreversible Small Peptidomimetics
 |  |  | ||
| Andrew A. Sproul, PhD | Carol M. Troy, MD, PhD | Michael Shelanski, MD, PhD |
Caspases are a family of cell death proteases that have been associated with neurodegenerative disorders since their initial identification. Broad-spectrum caspase inhibitors were proposed as possible neurodegenerative therapeutics, but significant toxicity occurs when such inhibitors are used for longer than 2 weeks, and the need for specific inhibitors targeting only the relevant caspase(s) was identified more than a decade ago. Most caspase inhibitors have been designed to target the active sites of each caspase based on combinatorial libraries. However, the active sites are highly similar across caspases, and the tetra- and pentapeptides used to target individual caspases have failed to distinguish between caspases. Most caspase inhibitors marketed as specific have been found to be non-specific at the functional level, providing data about caspases in general, not about specific caspases. Additionally, the interpretation of data obtained using these inhibitors has been complicated because some caspases have non-apoptotic functions that benefit the brain, such as regulating neurogenesis and synaptic activity. Therefore, prior studies using agents that target multiple caspases may have manipulated pathways beyond apoptosis, confounding interpretations of whether caspase inhibition is associated with therapeutic benefits.

Figure 7. LJ2a and LJ3a prevent dendritic spine loss induced by Aβ oligomers in hippocampal primary cultures. Swiss mice hippocampal neurons (E18) were cultured for 3 weeks in microfluidic chambers (20,000 neurons/chamber). Then neurons were pre-treated, or not, for 1 h with the indicated concentration of LJ2a or LJ3a, and treated for 6 h with 100 nM of monomeric Aβ (Aβ mono) or 100 nM of Aβ1-42 oligomers ([Aβ]n) then fixed and permeabilized for (immuno)staining with anti-MAP2, Phalloidin, anti-actin F, and anti-Bassoon. Microfluidic chambers were analyzed by fluorescence microscopy by counting phalloidin clusters affixed to MAP2 and Bassoon on hippocampal dendrites. a Representative micrograph of triple stained dendrites after 6 h in the presence (lower panel, [Aβ]n) or absence (upper panel, Co.) of Aβ oligomers. b, c. Quantification of dendritic spines in hippocampal neurons treated with LJ2a (b) or LJ3a (c) as indicated. Both inhibitors show synaptoprotective effects at submicromolar concentration. Histograms represent means (±SD) of 3 independent experiments (****p value ‹0.0001).
Studies from Taub labs and others have implicated caspase-2 as a key modulator of pathology in Alzheimer’s Disease (AD). We have shown that genetic knockdown or knockout provides protection against synaptic loss and neurodegeneration in mouse models of AD. While genetic approaches provide proof-of-concept we also want to develop tools for inhibiting caspase-2 activity that could potentially be developed as therapeutics. In a study recently published in Cell Death & Disease, in collaboration with Etienne Jacotot of Inserm (formerly a Schaefer fellow at Columbia), and Taub colleagues Drs. Michael Shelanski and Andrew Sproul, we have characterized new peptidomimetics, harboring non-natural modifications at the P2 position and an irreversible warhead (shown to be safe in humans). Enzyme kinetics show that these new compounds, such as LJ2 or its specific isomers LJ2a, and LJ3a, strongly and irreversibly inhibit caspase-2 with genuine selectivity. Particularly, LJ3a is highly selective for caspase-2 (946 times less efficient on caspase-3), far above previously described caspase-2 inhibitors. Additional enzyme kinetics experiments with human recombinant caspase-1, caspase-6, cathepsin-B, cathepsin-L, cathepsin-D, thrombin, plasmin, trypsin, kallikrein-1, -6, and -8 indicate that LJ2a and LJ3a have very limited or no effect on these enzymes. We further show that these potent and selective caspase-2 inhibitors have strong effects in several biological models, including protection against cell death induced by microtubule destabilization, and inhibition of synapse loss in primary hippocampal neurons treated with β-amyloid oligomers where submicromolar concentrations of LJ2a and of LJ3a prevent synapse loss, indicating a potential for further investigations in AD treatment.
Carol M. Troy, MD, PhD
Professor of Pathology and Cell Biology and Neurology (in the Taub Institute for Research on Alzheimer's Disease and the Aging Brain) at CUIMC
cmt2@cumc.columbia.edu
 |  |  | ||
| Yian Gu, MD, MS, PhD | Stephanie Cosentino, PhD | Yaakov Stern, PhD |
Dementia with Lewy bodies (DLB) is the second most common form of dementia following Alzheimer’s disease (AD). Several studies have reported a higher estimated cost of care associated with DLB compared to AD, yet our understanding of healthcare utilization and costs in DLB continues to be limited. While administrative databases are a rich source of information on healthcare use and costs, issues related to the substantial under-diagnosis, missed diagnosis, or mis-diagnosis of DLB, AD, and other dementias pose significant challenges when analyses rely solely on claims data. Additionally, though pathological confirmation is a gold standard of disease diagnosis, the question of whether clinical diagnosis and pathology-confirmed diagnosis are associated with healthcare costs has yet to be examined.

To explore some of these gaps, our latest work, co-authored by Taub colleagues Drs. Yian Gu and Stephanie Cosentino, represents the first known study to examine cost of care in autopsy confirmed DLB patients. Here, in collaboration with lead author Dr. Carolyn Zhu (Mount Sinai; not pictured), we analyzed healthcare expenditures in the last 5 years of life in a cohort of patients from the Predictors 2 study that had been clinically diagnosed with AD or DLB and had autopsy confirmed diagnosis of pure-AD, pure-DLB, or AD+DLB. As recently reported in the Journal of Alzheimer’s Disease, without considering the underlying pathology, we found that patients clinically diagnosed with DLB had higher expenditures than those clinically diagnosed with AD. When we looked at pathology, we found that expenditures were substantially higher in patients with mixed AD+LB pathology compared to those with pure-AD and pure-DLB pathologies. A closer look at the interaction between clinical and pathologic diagnoses showed that expenditures were particularly high in patients with mixed AD+LB pathologies, especially in those clinically diagnosed with DLB.
Our results point to the gaps in our understanding of healthcare costs in DLB and highlight the importance of having both clinical and pathology diagnoses in examining healthcare costs. With the high prevalence of DLB in an aging population and extremely high societal burden of healthcare costs, it is critical to improve current understanding of costs of care among patients with DLB in order to inform public policies and clinical decision-making, as this will ultimately improve the quality of patient care.
Yaakov Stern, PhD
Florence Irving Professor of Neuropsychology (in Neurology, Psychiatry, the Sergievsky Center, and the Taub Institute)
ys11@cumc.columbia.edu

