Columbia University
Irving Medical Center
Neurological Institute
710 West 168th Street, 3rd floor
(212) 305-1818
Featured Research
IN THE LAB:
Asa Abeliovich, MD, PhD

Asa Abeliovich, MD, PhD
The overall goal of the research program in our laboratory is to develop effective therapeutic strategies for Parkinson's disease (PD) and Alzheimer's disease (AD). There are two interrelated themes to these research efforts:
I. One focus is the pursuit of molecular and cellular mechanisms that underlie AD and PD. As a starting point, we have taken recent human genetics findings, particularly genetic risk factors associated with the common, non-familial forms of these disorders. Jessie Chang, a post-doc in the lab, is investigating how common variants at the end (3' untranslated region) of the aSynuclein gene may lead to an increase in PD risk. She is pursuing the hypothesis that these variants can modify aSynuclein RNA stability in neurons, and is developing screening tools to identify regulatory elements.
 Members of the laboratory, from left to right, Charles Lin, Luis Miguel Oliveira, Guy Ludwig, Jessie Chang, Asa Abeliovich, Ralitsa Petrova, Herve Rhinn, and Keiichi Inoue.
|
Within this same focus, another project regards the role of several PD genetic risk factors, including RAB7L1 and LRRK2, in the context of intracellular trafficking to the lysosome, pursued in collaboration with Taub colleagues Lorraine Clark and Scott Small. Post-doc Luis Oliveira is investigating the role of AD familial mutations (such as in Presenilin) or AD genetic risk-associated genes, such as APOE4, on APP processing. This work builds on transcriptome-wide gene expression and genomic studies that are part of a long-term collaboration between Herve Rhinn, a scientist in the group, as well as Rong Cheng and Joe Lee of the Taub Institute.
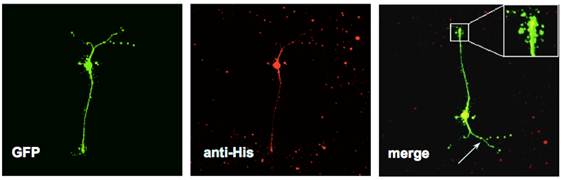 Primary rodent neurons (labeled with GFP, in green) expressing a mutant form of the Parkinson's gene LRRK2 (labeled with a His-tag, in red) display reduced neurite length and complexity. |
II. A second general focus of the lab is the development of novel model systems in which to study disease mechanisms and test potential lead therapeutics. Keiichi Inoue, a post-doc in the lab, is pursuing the generation of human neurons from patient fibroblasts, including patients with familial Presenilin mutations, in a long-term collaboration with Taub colleagues Gil Di Paolo and Scott Small. The goals are to identify disease-related changes and underlying drug targets, and to, ultimately, test therapeutic leads. Ralitsa Petrova, a postdoc in the lab, is developing tools to integrate newly-generated neurons into the brain in vivo, in collaboration with Rafael Yuste at Columbia University. Such an approach may be useful for disease modeling, as well as regenerative medicine approaches.

