Columbia University
Irving Medical Center
Neurological Institute
710 West 168th Street, 3rd floor
(212) 305-1818
Featured Research
In the Lab:
Natura Myeku, PhD
 Natura Myeku, PhD
Natura Myeku, PhDThe main focus of my research is to understand the mechanisms of deficient protein degradation in Alzheimer's disease (AD) and other disorders that have the accumulation of toxic proteins as a common feature. In particular, we are interested in studying the ubiquitin proteasome system (UPS), which is the principal pathway for protein turnover in eukaryotic cells. We use unique transgenic mouse models of AD and the UPS to identify pathways associated with dysregulated clearance of tau and other proteins during disease progression. Understanding what causes the UPS to become defective, as well as its role in disease pathogenesis, can help us identify novel therapeutic targets for prevention or treatment.
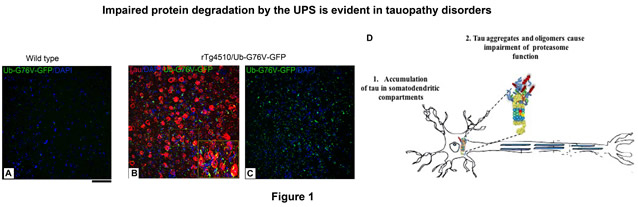
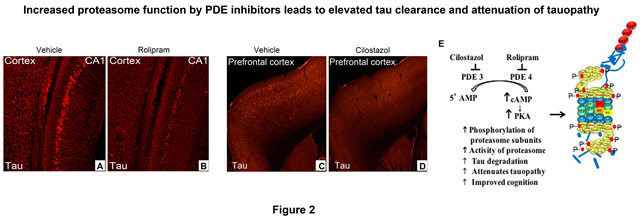
During my postdoc in the laboratory of Dr. Karen Duff, we demonstrated that, in different tauopathy models, the UPS protein clearance collapses as tau aggregates directly associate with proteasome complex, thereby obstructing the entry channel of proteasomes and overall protein proteolysis (Fig. 1). Moreover, fully functional proteasomes resemble pathological proteasomes when incubated with tau oligomers, demonstrating that this proteolytic complex is directly implicated in the disease pathogenesis in tauopathy, and most likely in other proteotoxic diseases, as well. This finding led to the application of a therapeutic strategy discovered during my graduate studies that phosphorylation of proteasomes has a protective effect on dysfunctional proteasomes. In a relevant mouse model, we showed that impaired proteasome function can be rescued by clinically relevant drug called rolipram, leading to clearance of tau, attenuation of tauopathy, and improved memory in tauopathy mice (Fig. 2A and B).
|
Figure 1. A) UPS reporter (wild type) mice show negligible accumulation of a reporter protein, Ub-G76V-GFP (green), as proteasomes clear it swiftly. B and C) Triple transgenic (rTg4510/Ub-G76V-GFP) mice show prominent accumulation of GFP (green) puncta and human tau (red), indicating that the UPS clearance is impaired in tauopathy. D) Illustration of how accumulated tau can obstruct and impair proteasomes. .
|
As an independent investigator, my work, thus far, is focused on translational science, whereby basic science knowledge is translated into novel therapeutic strategies. One approach is to identify which phosphodiesterase (PDE) inhibitor can be used or redirected as a therapy against accumulation of toxic tau and other proteins. Our current work has uncovered that several PDE inhibitors act as proteasome activators in vivo (Fig. 2). Repurposing this class of drugs for treatment of Alzheimer's disease can have rapid clinical impact in patients, since several PDE inhibitors are approved by the FDA for treatment of other chronic disease (e.g. PDE3 inhibitor, cilostazol [Fig. 2D]).
|
Figure 2. Immunofluorescence labeling of human tau (red) in the first study, A) vehicle and B) rolipram treated rTg4510 mice. And in the second study, C) vehicle and D) cilostazol treated rTg4510 mice. E) Illustration of the mechanism of proteasome activation/phosphorylation by PDE inhibitors.
|
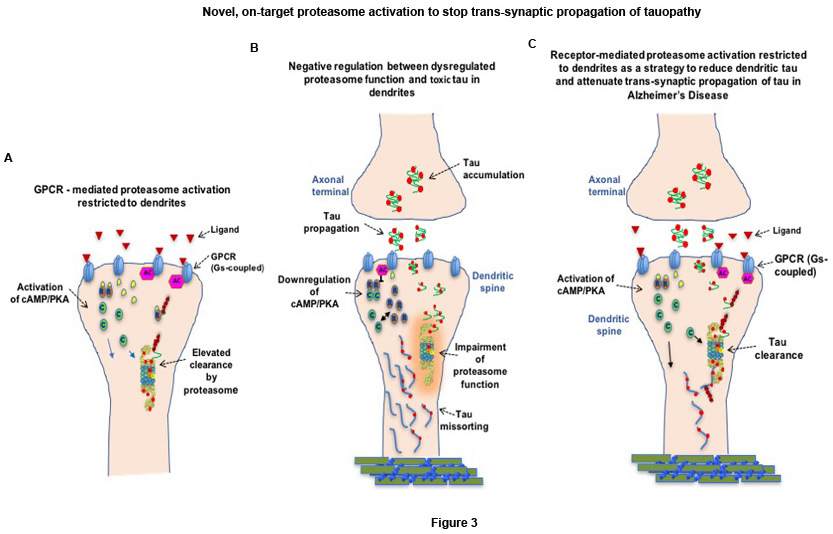
Our current collaboration with Dr. Karen Duff is aiming to identify a better on-target approach to combat early stages of AD, right when toxic tau species start accumulating in synapses and then propagate trans-synaptically throughout the affected brain areas. For this project, we aim to use relevant GPCR-(G-protein-coupled receptors-) targeted drugs, which are the largest class of therapeutic agents on the market. We are testing whether GPCR-mediated proteasome activation restricted to dendrites can destroy traveling tau when internalized in the post-synaptic compartments, and stop trans-synaptic propagation of tau along anatomical projections (Fig. 3A and C). In vivo experiments are underway to test if the spread of tauopathy can be stopped. In addition, the molecular mechanisms involved in site-specific degradation of tau are being tested in collaboration with the laboratory of Dr. Ulrich Hengst, in an in vitro paradigm using microfluidic chambers that will enable the visualization and manipulation of synapses in isolation.
|
Figure 3. Illustration of our hypothesis A) GPCR-mediated proteasome activation restricted to dendrites. B) Tau species that propagate trans-synaptically may "choke" synaptic proteasomes and exacerbate synaptic dysfunction. C) Therapeutic strategy to stop trans-synaptic propagation of tauopathy by enhancing proteasome-mediated tau clearance in the post synaptic compartments.
|
My lab projects will continue to study the functionality and the implication of UPS in the pathogenesis of neurodegenerative diseases. Furthermore, plans are underway to develop a cell-based screening assay that we can utilize to discover and develop small molecules as proteasome activators for therapeutic and research purposes.
Natura Myeku, PhD
Assistant Professor of Pathology and Cell Biology
nm2631@cumc.columbia.edu