Columbia University
Irving Medical Center
Neurological Institute
710 West 168th Street, 3rd floor
(212) 305-1818
Featured Research
IN THE LAB:
Ismael Santa-Maria Perez, PhD
 Ismael Santa-Maria Perez, PhD
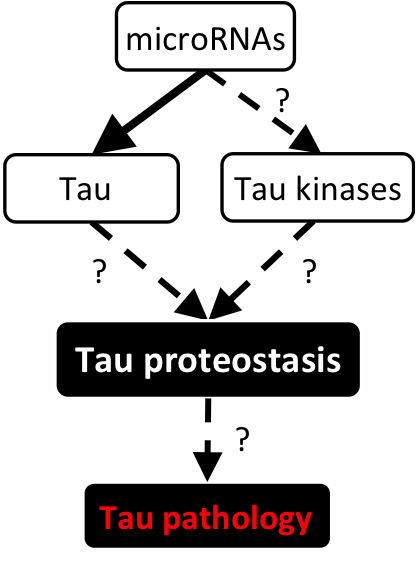
Ismael Santa-Maria Perez, PhDResearch in our laboratory is focused on understanding the mechanisms maintaining or altering tau proteostasis in neurons, and their relevance in aging and Alzheimer's disease (AD). Intracellular accumulation of neurofibrillary tangles of hyperphosphorylated misfolded tau proteins is one of the main hallmarks in many neurodegenerative diseases, including AD. Little is known about the mechanisms underlying tau dysfunction and neurofibrillary degeneration but a dysfunctional regulation of protein expression has been proposed to participate in the pathogenesis of AD and related tauopathies. In addition, it is unclear whether disturbances in the levels of microRNAs or other molecules that directly regulate tau proteostasis contribute to the pathogenesis of AD. Hence, investigating how certain microRNAs impact tau synthesis and accumulation in the disease is currently the main interest of our laboratory. We employ a variety of biochemical, cell culture, and transgenic animal approaches and techniques in our research, in close collaboration with other members of the Taub Institute, including Brian McCabe, Michael Shelanski, and Karen Duff.
Our research has shown a specific microRNA, miR-219, is downregulated in AD in autopsy brain tissue from the New York Brain Bank (courtesy of Jean Paul G. Vonsattel & Etty Cortes). Using mammalian cell cultures and a novel transgenic Drosophila model, we have also found that miR-219 directly binds to the tau mRNA 3' untranslated region (UTR) and silences its expression at the post-transcriptional level. Moreover, in transgenic Drosophila flies that express human tau, we observed that miR-219 reduced tau toxicity, suggesting that miR-219 is part of a vital regulatory mechanism that prevents the deposition of tau (Figure 1).
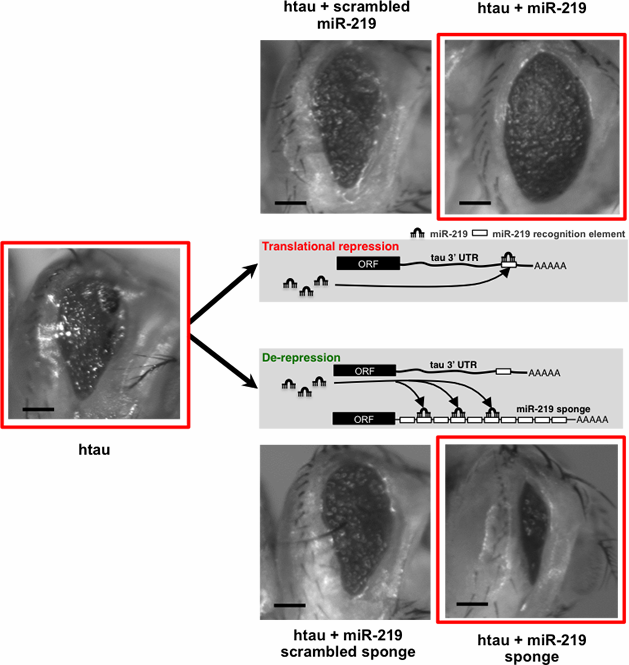 Figure 1: Modulation of tau toxicity by miR-219 in Drosophila. Images show tau induced phenotype in the fly eye which is modulated in the presence or absence of miR-219.
|
Strikingly, our bioinformatics analysis indicates miR-219 is also predicted to target Tau tubulin kinase 1 (TTBK1), Calcium/calmodulin-dependent protein kinase II (CaMKII), AMP-activated protein kinase (AMPK), and Glycogen synthase kinase 3 beta (GSK3β), which are all implicated in the generation of abnormal hyperphosphorylated tau. Our central hypothesis is that downregulation of microRNAs leads to de-repression of a genetic program that increases expression of genes that drive tau toxicity (Figure 2).
|
|
|
Maria Eugenia Alaniz is leading the project using Drosophila melanogaster (fruit fly) as an animal model to continue exploring the role of microRNAs in tau pathogenesis. This work, in collaboration with Brian McCabe, aims to characterize microRNA regulation of the expression of tau and GSK3β and to demonstrate that microRNAs modulate tau neurotoxicity using various CRISPR-edited and other transgenic flies we have developed. Isabel Nelson is leading our efforts in addressing the role of microRNAs in tau proteostasis regulation in mammalian cellular systems, in neuronal cell lines and neuronal primary cultures. She is specifically focused on the question of how miR-219 levels are regulated and whether this microRNA targets other proteins modulating tau physiology and pathology. In addition, we all continue investigating the role of microRNAs in the post-transcriptional regulation of tau in mice. We are working in collaboration with Karen Duff to determine the extent to which tau 3'UTR-microRNA interactions influence tau toxicity in a transgenic mouse model of AD-related tauopathy.
 Members of the Santa-Maria Perez Laboratory include, from left to right, Isabel Nelson, Ismael Santa-Maria Perez and Maria Eugenia Alaniz.
|
Additionally, in order to rule out which other molecules might directly regulate tau proteostasis and neuronal cell death, we are working in collaboration with Ying Jean and Carol Troy on a project involving CUGBP2, an RNA binding protein identified by genome wide association studies (GWAS) in late-onset AD.