Columbia University
Irving Medical Center
Neurological Institute
710 West 168th Street, 3rd floor
(212) 305-1818
Featured Research
Quantitative Neuroimaging Laboratory:
Qolamreza R.
Razlighi, PhD
 Qolamreza R. Razlighi, PhD
Qolamreza R. Razlighi, PhDThe Quantitative Neuroimaging Laboratory (QNL) is a neuroimage processing research lab housed in the Cognitive Neuroscience Division of the Taub Institute and the Department of Neurology at Columbia University Medical Center. The main focus of the QNL is to develop techniques to quantify structural and functional brain images, which allow researchers and clinicians to detect brain-based effects that are normally beyond the sensitivity and specificity of commonly-used detection methods in the field. We aim to achieve this goal by utilizing state-of-the-art signal and image processing tools, as well as mathematical and statistical methods. The QNL is also affiliated with Columbia's Department of Biomedical Engineering and the Gertrude H. Sergievsky Center.
The main research project in QNL is to investigate the physiological and neural bases of the fMRI-BOLD deactivations or negative hemodynamic response during task performance. Almost any goal-oriented task performance generates negative BOLD response in some regions of the brain. While the evidence for the physiological and neural bases of positive fMRI-BOLD activation is emerging in the neuroimaging field, little or none is known about its negative counterpart. In theory, under the perfect cerebral auto-regulated condition, in which the brain's total input blood flow is kept constant, the brain cannot generate positive hemodynamic response without inducing negative hemodynamic response in other regions. However, how such a dynamic is regulated in the brain, how the brain decides what regions to be deactivated, and where in the brain this process takes place are all currently unknown.
One of the major challenges in such investigations is the use of the same methods and techniques that have been used in the field for investigating positive BOLD response, while none of them have, in fact, been evaluated or optimized for analyzing the negative BOLD response. QNL, unlike other laboratories investigating negative BOLD response, is well equipped to either modify the existing methods or to introduce completely new methods that are more suitable for analyzing negative BOLD response. For instance, we have come to the realization that negative BOLD response is more sensitive to the time delay in the slice acquisition, thus the existing slice timing correction techniques are bound to fail in investigating negative BOLD response. Therefore, we introduced a completely novel method to perform optimal slice timing correction. This new method is also beneficial in analyzing the positive BOLD response, thus it has been shared with the neuroimaging community for analysis of fMRI-BOLD, in general. We are also currently extracting the hemodynamic impulse response for negative BOLD from different nodes of the default mode network. In addition, we are investigating the linearity of the negative BOLD response and the conditions on which such linearity holds. We have also shown that extraction of hemodynamic impulse response function can be inaccurate if sub-optimal spatial normalization methods are used, thus we introduced a new method to improve the accuracy of spatial normalization in fMRI data analysis.
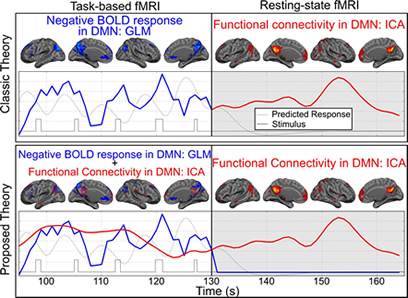
Figure 1
Negative BOLD response has some specific characteristics which can be used in studies investigating brain neurophysiology and functionality. For instance, in a recent manuscript, we used attention-specificity of the negative BOLD response to show that resting-state functional connectivity and task-based deactivation in the default mode network regions are two overlapping but separate neurophysiological processes. Furthermore, we hypothesized that resting-state functional connectivity network provides the underlying infrastructure for the task-based co-activation networks. Using attention-specificity of the negative BOLD response, we have demonstrated that we can induce a significant reduction in the blood flow of the default mode network regions without altering the underlying functional connectivity. Our hypothesis, if proven, is introducing a paradigm shift in the current understanding of how brain large scale networks function and what is the relationship between resting-sate functional connectivity networks and task-based co-activation networks. This has been illustrated in figure 1.
Task-specificity of the negative BOLD response is another interesting characteristic that we are attempting to utilize to understand how brain regulates or redistributes the available metabolic resources between regions to perform different tasks. Our preliminary data indicates that the task-based deactivation in the brain regions not only do not take place randomly, but also they are more precise than activations. In other words, the brain knows exactly what type of a task is being performed and what regions are not going to be involved in such a task. Therefore, the brain inhibits the resting activities only on the regions that are not going to be involved in the task performance. These findings can be used as preliminary evidence for a new subsystem in the brain that regulates or redistributes metabolic resources. Since such systems exist in pretty much any man-made computing machine and are called operating systems, we also named this subsystem the brain operating subsystem (BOSS).
Within my Quantitative Neuroimaging Laboratory, current members and their projects include:
 Members of the Quantitative Neuroimaging Laboratory include, from left to right: Krista Polly, Elizabeth Meyer, David Parker, Sam Bou-Zeid, and Xueqing Liu. |
Sam Bou-Zeid, a postdoctoral research scientist, is developing a new reconstruction technique to analyze amyloid PET images.
David Parker, a PhD candidate from biomedical engineering, is devoting his PhD research on slice-timing correction and motion correction and their interaction in fMRI data analysis.
Xueqing Liu, a PhD student from biomedical engineering, is focusing on simulating the effects of physiological fluctuations on BOLD-fMRI signal.
Krista Polly, a neuroscience MSc student from Teachers college, is focusing on developing a new method for extracting intracranial volume from the T1w structural image.
Hengda He, a visiting scholar from China, focuses on the enhancement of the region-based spatial normalization technique that we have introduced to improve the accuracy of the spatial normalization in fMRI data analysis.
Qolamreza R. Razlighi, PhD
Assistant Professor of Neuroimaging (in Neurology and the Taub Institute for Research on Alzheimer's Disease and the Aging Brain)
Adjunct Assistant Professor in Biomedical Engineering
Quantitative Neuroimaging Lab
qr2108@cumc.columbia.edu

