Columbia University
Irving Medical Center
Neurological Institute
710 West 168th Street, 3rd floor
(212) 305-1818
Featured Research
In the Lab:
Diego E. Berman, PhD
 Diego E. Berman, PhD
Diego E. Berman, PhDA common emerging theme in the field of age-related neurodegenerative disease research is that of endosomal dysfunction. In the case of Alzheimer's disease (AD), this is particularly important, as the endosomal compartment of the cell is one of the main sites in which the amyloid precursor protein (APP) is processed, initiating a cascade of events leading to the production and accumulation of a number of neurotoxic protein fragments, including beta-amyloid.
Research in our laboratory focuses on understanding the molecular and cellular biology of a master conductor of protein trafficking and regulator of endosomal biology: the retromer complex. The retromer complex is a multiprotein assembly that transports cargo proteins out of the endosome into the trans-Golgi network and the plasma membrane. In neurons, the retromer pathway plays a critical role in the cellular trafficking of APP, by keeping it away from endosomes and thus controlling the production of its neurotoxic fragments. In recent years, our lab has shown that levels of the retromer complex are decreased in the cortex of AD patients. In parallel studies, in vitro cell culture experiments show that reducing retromer levels by using small interfering RNA (siRNA) leads to an increased production of beta-amyloid, while, on the other hand, retromer overexpression leads to decreased amyloid accumulation. Moreover, our research shows that retromer deficient transgenic mice exhibit altered behavior and an increased amyloid burden in the brain. These data, together with that of other laboratories, led us to the hypothesis that increasing the levels and stability of the retromer complex would improve its function and, in turn, rescue deficiencies in the endosomal trafficking and transport machinery. Furthermore, this body of evidence validates endosomal transport as a "cell biological" target for drug discovery.
Recently, in collaboration with Dr. Greg Petsko now at Weill Cornell Medical College, our lab identified a new family of small molecules dubbed 'retromer pharmacological chaperones' that stabilize the interaction between members of the retromer complex and enhance its function in neurons. By using a wide array of multidisciplinary tools, including cell biological techniques, confocal microscopy, and biochemistry, we showed that the retromer chaperones keep APP away from the endosome and reduce the production of the beta-amyloid peptide.
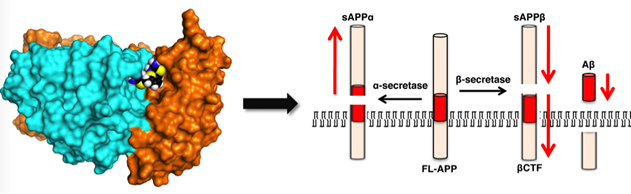 Space-filling diagram depicting a retromer chaperone molecule docked at the interface pocket between the retromer proteins Vps29 (light blue) and Vps35 (orange). Stabilization of the complex by the chaperone leads to decreased APP processing and beta-amyloid accumulation
|
While these findings have established the use of small molecules to improve retromer complex function, the road to developing a successful drug that will display positive pharmacological dynamics in vivo might take several years of medicinal chemistry and biological testing and screening. For this reason, our lab is currently developing an alternative, biologically-based approach to increase retromer levels and function in vivo: the overexpression of retromer by the use of recombinant AAV (associated adenovirus) technology. We are now establishing novel retromer-AAV tools to be used for research and potential therapeutics, as this viral delivery system—recently approved for clinical applications—can bypass the obstacles that a small molecule would encounter within the organism (i.e. low absorption rates, degradation, toxicity, lack of target/organ specificity, blood brain barrier permeability, etc). As can be seen in the picture below, we have successfully infected and expressed a recombinant human form of the Vps35 protein of the retromer complex by injecting retromer-AAV vectors into the pars compacta region of the substantia nigra in the rat brain. In collaboration with the Stem Cell and Cellular Models platform directed by Dr. Andrew Sproul, we are also testing the effect of our various novel retromer overexpression viral tools on iPSC-derived human neurons. Ongoing studies in the lab are testing the efficacy of these retromer viral tools in the improvement of retromer function in vivo, which we hope will translate into improved endosomal function and a reduction in AD pathophysiology.
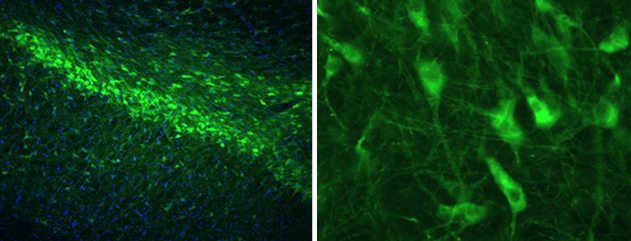 Overexpression of human VPS35-GFP in the rat brain
by the use of a recombinant AAV9 vector
|
Another area of interest in the lab has been the identification of novel retromer-related genes involved in the generation of AD-like pathologies. In collaboration with Taub faculty member Dr. Christiane Reitz, we have identified a Late-Onset AD (LOAD) variant in an endo-lysosomal-compartment associated gene, the Ceroid-lipofuscinosis, neuronal 5 (CLN5), by using genome wide association studies (GWAS) and whole exome sequencing (WES). The CLN5 gene is implicated in Neuronal Ceroid Lipofuscinoses (NCL), a group of neurodegenerative disorders that affect children. Our data shows that this mutant CLN5 variant results in aberrant post-translational modifications, such as abnormal folding and defects in glycosylation patterns, leading to mis-localization and entrapment of CLN5 in the endoplasmic reticulum, and overall cellular protein trafficking defects. We are also actively studying the role of a recently identified AD-linked variant of the sortin nexin 1 (SNX1) protein, a member of a large family of hydrophilic proteins that interacts with retromer and with a variety of membrane receptors involved in intracellular trafficking.
A new and exciting exploratory area of research in the lab is the characterization of the role of the retromer complex in Amyotrophic Lateral Sclerosis (ALS). In collaboration with Drs. Serge Przedborski and Francisco Lotti from the Motor Neuron Center, we are currently studying the role of retromer dysfunction in non-cell autonomous motor neuron death, focusing specifically on the role of retromer trafficking in healthy and diseased (ALS) spinal cord astrocytes.
 From left to right, Milan Kothiya, Diego Berman (PI), Yasir Qureshi, and Vivek Patel.
|
Diego E. Berman, PhD
Assistant Professor of Pathology & Cell Biology
db2146@cumc.columbia.edu

