Columbia University
Irving Medical Center
Neurological Institute
710 West 168th Street, 3rd floor
(212) 305-1818
Featured Research
IN THE LAB:
Tae-Wan Kim, PhD
 Tae-Wan Kim, PhD
Tae-Wan Kim, PhDThe focus of our laboratory is to discover and validate novel therapeutic targets for Alzheimer's disease (AD) using genetic and chemical tools. Our main approach is first to establish phenotypic assays that recapitulate key pathogenic phenotypes, or disease-relevant physiology. We employ physiologically characteristic brain cells, including stem cell-derived neurons, and perform unbiased high throughput compound screening for the identification of chemical probes that modulate cellular phenotype(s) of interest. We then conduct target identification and validation studies to understand the molecular and cellular basis of the compound's action in modifying AD-relevant phenotypes. In addition to target exploration, our phenotypic screening approach also serves as an alternative way to discover new drug activities for AD from a library of known drugs ("Drug Repurposing"). Thus, our approach not only identifies chemical tools to probe new targets/pathways but also provides the opportunity to identify promising drug candidates. Ultimately within the next few years, we would like to translate our laboratory discovery into a successful drug development program for human clinical testing.
The lab is currently conducting the following projects:
Our first project is to identify small molecule enhancers of apolipoprotein E (apoE) via new mechanisms of action through high throughput, phenotypic compound screening. ApoE is mainly secreted from astrocytes in the brain and has been involved with several AD-relevant brain functions such as neuroinflammation and the clearance of amyloid β-peptide (Aβ). Despite the central role for apoE in AD, detailed knowledge of how apoE expression/secretion is regulated in astrocytes is essentially unexplored. Our present study is to conduct cell-based high throughput screening (HTS) assays to identify novel compounds that increase the secretion of apoE from primary human astrocytes. We use a primary human cell system for our HTS campaign that best demonstrates physiological relevance and adaptability for HTS. Our screening efforts, to date, have already yielded several interesting compounds and their corresponding cellular targets, which will allow us to elucidate entirely new regulatory mechanisms for brain apoE that can be used to devise new therapeutic strategies. Our study will also provide an important basis for developing ways to manipulate the functionality of apoE in vivo for therapeutic purposes. For instance, we hypothesize that increased levels of the apoE3 isoform may confer beneficial effects in AD by promoting Aβ clearance and suppressing neuroinflammation (vs. apoE4 that confers a higher risk for AD). Thus, our newly-discovered, apoE-enhancing small molecules could potentially serve as a foundation for developing new AD therapeutics. This project is being led by Gina Finan in collaboration with Dr. Charles Karan and his colleagues at the High-Throughput Screening Core, Columbia Genome Center, and Dr. Nan Wang of the Department of Medicine at CUMC.
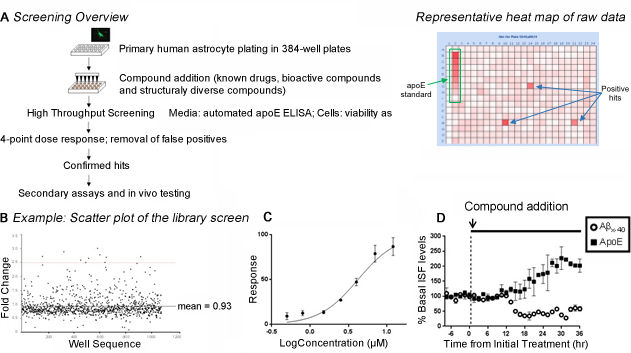 High throughput screening (HTS) for apoE-enhancing small molecules. (A) Workflow of small molecule screening for apoE enhancers in primary human astrocytes. Astrocytes were plated in 384-well plates and subjected to HTS of the chemical libraries using optimized apoE sandwich ELISA. (B) Scatter plot of the representative library screen (1280 compounds) assayed via apoE ELISA at 10µM in 384-well plates, using primary human astrocytes. "Hits" are defined as a fold change >2.5 (~ 4 standard deviations), red dotted line. Mean fold change of library = 0.93. (C) Dose response curve of one of the hit compound. (D) Effects of a hit compound on ISF Aβx-40 and apoE levels using in vivo microdialysis. Following establishment of a 6 hour baseline ISF level for Aβx-40 and apoE, mice were administered AY-9944 via reverse microdialysis and ISF Aβx-40 and apoE levels were assessed for an additional 36 hours. AY-9944 significantly increased ISF apoE levels (201.8 ± 31.3%, n=2) compared to baseline. This hit compound decreased ISF Aβ levels (58.1 ± 10.7%, n=2) compared to baseline.
|
Gina Finan is currently also a lead investigator on developing phenotypic assays for tau, which is our second project. Our laboratory has previously established a neuronal model derived from mouse embryonic stem cells that is suitable to HTS. Using this model system, we have identified an FDA-approved compound (that is being used for a rare infectious condition) that can reduce the levels of pathological tau and also confer protection against synapse-impairing activity of Aβ. We found that this compound can rescue learning and memory impairments in a Tg2576 mouse model of AD and we are currently attempting to identify a cellular target(s) for the compound's novel bioactivity.
Laura McIntire, recently promoted to Assistant Professor, is leading our third project on the regulation of β-site amyloid precursor protein (APP)-cleaving enzyme 1 (BACE1). BACE1 mediates the first and rate limiting proteolytic step leading to production and eventually deposition of Aβ in brain. BACE1 is a major drug target for the treatment of AD and clinical development of BACE1 inhibitors is currently being intensely pursued. Our previous studies using proteomics and cell biological approaches discovered several cellular factors that regulate BACE1 including sortilin and sorting nexin 6. Complementary to these approaches, this project employs a chemical biology strategy to further explore the mechanisms of cellular BACE1 regulation. To elucidate how BACE1 is regulated in the brain using mechanistic small molecule probes, we first developed a novel reporter-based cellular assay for detection of the soluble APP fragment proteolytically liberated from cell-associated APP by BACE1 and conducted a high throughput cell-based screen. We identified small molecules that can inhibit BACE1-mediated cleavage of APP but do not directly act though catalytic inhibition of the BACE1. Using these chemical probes, our laboratory is trying to understand the mechanism of BACE1 regulation by identifying cellular target(s) of these novel BACE1-modulating compounds.
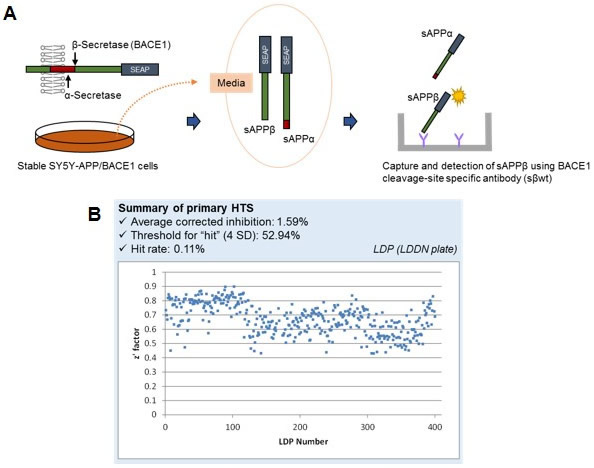 Characterization of cell-based HTS assay for detection of APPβ in neuronal cell line. (A) Schematic illustration of the assay. The engineered stable SY5Y transfectants expressing BACE1-GFP and secreted alkaline phosphatase conjugated APP (SEAP-APPwt; SY5Y-APP/BACE1 cells, BGWT8 line) are grown on standard cell culture plates. SEAP-APPwt is cleaved by BACE1 (β-secretase) as well as α-secretase to release large soluble fragments sAPPβ and sAPPα, respectively. Cell culture media containing these fragments is harvested and SEAP-sAPPβ (but not SEAP-sAPPα) is selectively captured using 384-well plates coated with the β-cleavage-specific antibody (sβwt) subjected to ELISA detection. (B) Summary of primary screening, and scatter chart of screening plate. Z' factor values were constantly around or higher than 0.5, indicating that the screening was done in a robust manner. The total of ~140,000 compounds were screened.
|
The fourth project in the lab, also led by Laura McIntire, is to explore the potential for therapeutic targets in phosphoinositide lipid pathways. Our long-time interest has been to identify the fundamental biochemical and cellular defects associated with familial forms of AD (FAD) and understand the genotype-to-phenotype transition in the context of FAD-linked mutations in the presenilin genes. These investigations led to the discovery of FAD-associated alterations in phosphoinositides, specifically phosphatidylinositol 4,5-bisphosphate [PI(4,5)P2]. This project is investigating the roles for key mediators in phosphoinositide signaling with an emphasis on synaptojanin 1 (Synj1), which tightly controls PI(4,5)P2 levels at the plasma membrane and synapse. Synj1 is a brain-enriched lipid phosphatase responsible for breakdown of PI(4,5)P2, shown to play a role in synaptic vesicle recycling and receptor trafficking in neurons. In collaboration with Drs. Gilbert Di Paolo and Ottavio Arancio, we demonstrated that genetic reduction of Synj1 inhibited the destabilizing effect of Abeta Aβ on PI(4,5)P2 and ameliorated Aβ-induced synaptic dysfunction and memory impairment in neurons and brains of AD mouse models. We are currently elucidating the cellular and molecular mechanisms underlying Synj1 function in Aβ-induced synaptic alterations by determining relevant and critical functional domains of Synj1 as well as characterizing the novel molecular interaction of Synj1 with bridging integrator 1 (BIN1), the gene product of a recently discovered AD susceptibility gene.
 Members of the Kim Laboratory, from left to right, Tae-Wan Kim (PI), Meewon Park, Laura McIntire, Yoon Kim (a PhD rotation student), Gina Finan, and Francesca Garretti (a PhD rotation student).
|
Tae-Wan Kim, PhD
Associate Professor of Pathology and Cell Biology (in the Taub Institute)
twk16@cumc.columbia.edu

