Columbia University
Irving Medical Center
Neurological Institute
710 West 168th Street, 3rd floor
(212) 305-1818
Featured Research
In the Lab:
Philip L. De Jager, MD, PhD

Philip L. De Jager, MD, PhD
As a clinician-scientist, I have a broad approach which integrates rigorous cell and molecular biology experiments of human samples with cutting-edge computational methods to explore the onset and course of neuroinflammatory and neurodegenerative diseases that affect the human brain. In the course of these investigations, we also pursue fundamental human neurobiological questions, such as describing nine subsets of human microglial cells and defining their transcriptional signatures. A proponent of team-based science, I assemble a team for each project that is typically composed of an experimental biologist, a computational scientist, and a clinician-scientist. Each project is led by one laboratory member, with members taking on both leading and supportive roles on different projects.
I am the Chief of the Division of Neuroimmunology in the Department of Neurology which includes the Center for Translational & Computational Neuroimmunology (CTCN). The CTCN includes my laboratory as well as those of six independent investigators, and we are all members of the Taub Institute. My laboratory focuses primarily on the study of cognitive aging and Alzheimer’s disease as well as multiple sclerosis. As a translational scientist, I design projects which typically involve primary human material (blood, cerebrospinal fluid, or brain tissue) and/or model systems using human cells. Current projects in the laboratory are almost always collaborative with other investigators inside and outside of the CTCN. They include but are not limited to:
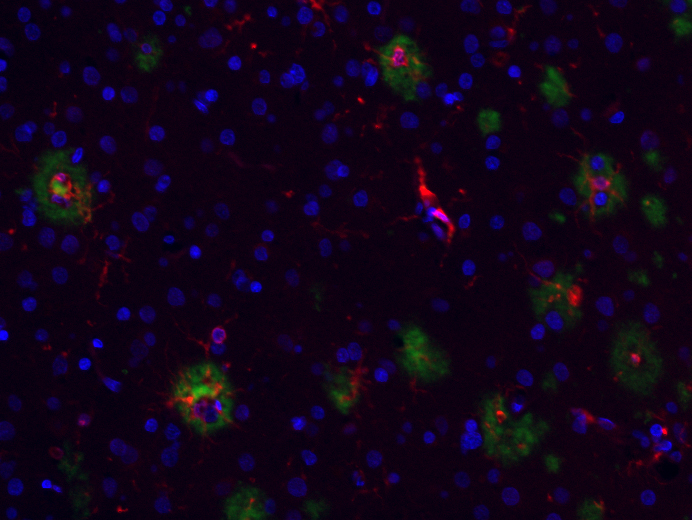 Figure: Photo of human microglia (red) in and around amyloid plaques (green) in the human cortex, courtesy of Dr. Mariko Taga.
Figure: Photo of human microglia (red) in and around amyloid plaques (green) in the human cortex, courtesy of Dr. Mariko Taga.
- Defining microenvironments in the human brain using single nucleus sequencing, spatial transcriptomics, and miniaturized proteomic profiling: exploring inter-cellular communication between discrete groups of specialized subtypes of astrocytic, endothelial, microglial and other cells.
- Assembling and dissecting protein complexes composed of Alzheimer-associated genes in microglia to identify the functional consequences of genetic risk factors.
- Defining the cellular composition of human cerebrospinal fluid at single cell resolution over the life course, in the context of inflammatory diseases, and in response to immunotherapies to elucidate disease and therapeutic mechanisms.
- Deconstructing the aging brain’s epigenome to map the sequence of events leading from health to disease as well as mechanisms that contribute to cognitive resilience in the face of pathology.
- Understanding the immune and CNS mechanisms leading to the onset of neuroinflammation in multiple sclerosis and the triggering of a secondary neurodegenerative process that produces most of the disability in this disease.
-
Applying machine-learning, graph theory and other analytic approaches to biological data to resolve the complexity of human neurobiology and neurodegenerative diseases.
I could not complete these projects if I worked in isolation, and they also rely on achieving the appropriate sample sizes of human material and involvement of specialists in different disciplines through collaborative networks such as the CZI Neurodegeneration Challenge Network, the NIA’s Accelerating Medicines Partnership for Alzheimer’s Disease, and the International Multiple Sclerosis Genetic Consortium to name just a few.
|
Members of the Center for Translational & Computational Neuroimmunology
|
I am honored to have been named Deputy Director of the Taub Institute for Research on Alzheimer’s Disease and the Aging Brain. I look forward to working with all of my colleagues within the Taub Institute to accelerate the dissemination of new laboratory techniques and analytic methods, as well as to facilitate the development and execution of new collaborative projects. When pursued by a team of investigators, the combination of novel tools with the rich specimen from the many human cohort studies at Columbia University Irving Medical Center will enable us to attack the most urgent questions that will inform the development of interventions to support brain (and spinal cord) health with advancing age.
Philip L. De Jager, MD, PhD
Weil-Granat Professor of Neurology (in Neurology, the Taub Institute for Research on Alzheimer's Disease and the Aging Brain and the Precision Medicine Initiative)
pld2115@cumc.columbia.edu


