Columbia University
Irving Medical Center
Neurological Institute
710 West 168th Street, 3rd floor
(212) 305-1818
Featured Research
IN THE LAB:
Carol M. Troy, MD, PhD

Carol M. Troy, MD, PhD
The work in my laboratory stems from my long-standing interest in understanding the molecular specificity of death pathways. Throughout the body there is homeostasis of life and death at the cellular level. In disease, where death is dysregulated in particular cells, there is alteration in the affected cells but not throughout the body. Thus, we need to identify specific targets that are altered in the disease state but are not required for normal cellular homeostasis. In our lab, we focus on the regulation and function of the caspase family of proteases in the mature nervous system. Best known as the executors of cell death, there is increasing appreciation that some caspase may also have non-apoptotic functions. Individual caspases cleave specific substrates at one or two cleavage sites. Cleavage can result in inactivation of a substrate, a change in the substrates activity, or target the substrate for ubiquitination and degradation. However, caspase cleavage of a substrate on its own does not degrade the cellular proteins. This places aberrant caspase activity as a potential therapeutic target. We are utilizing novel approaches to inhibit specific family members to dissect the function of each in the normal brain and in disease. We utilize in vivo and in vitro models to study both molecular pathways and therapeutic interventions.
 Members of the Troy Laboratory, from left to right, Ying Jean, Alexi Geevarghese, Elisa Canepa, Tess Kichuk, Maya Ramachandran, Maria Avrutsky, Gloria Hong, and Carol Troy. Not pictured: Maria Elena Pero and Sasha White.
|
Currently the lab is focused on the following projects:
Elisa Canepa is leading a project focused on the function of caspase-9 in cerebral ischemia. This work is in collaboration with Sander Connolly in the Department of Neurosurgery. The current project arose from our finding that caspase-9 was the first caspase activated in rodent models of cerebral ischemia, and that inhibition of caspase-9 activity, by intranasal delivery of Pen1-XBIr3––a novel cell permeant caspase-9 inhibitor we developed––provided morphologic and functional neuroprotection. As we looked more closely at the data, we realized that inhibition of caspase-9 significantly abrogated cerebral edema during stroke. Cerebral edema is the major cause of acute death from stroke and there are no molecular interventions to treat edema. To address the question of whether edema is caspase-9 mediated, we examined whether caspase-9 was increased in small blood vessels, the source of edema, during stroke. Surprisingly, we found that there is a large increase in caspase-9 protein expression in the endothelial cells. Elisa is now developing mice which will have inducible knockout of caspase-9 in endothelial cells or neurons to determine the cell specific function of caspase-9 during stroke. These animals will be studied with live imaging (in collaboration with Imre Bartos in the Marka Lab in Columbia's Department of Physics, who is developing software that will provide an objective analysis of mouse neurologic function, pre- and post-stroke), with immunohistochemical and biochemical techniques to study the contribution of caspase-9 in each cell type. Tess Kichuk and Alexi Geevarghese are assisting Elisa with her studies.
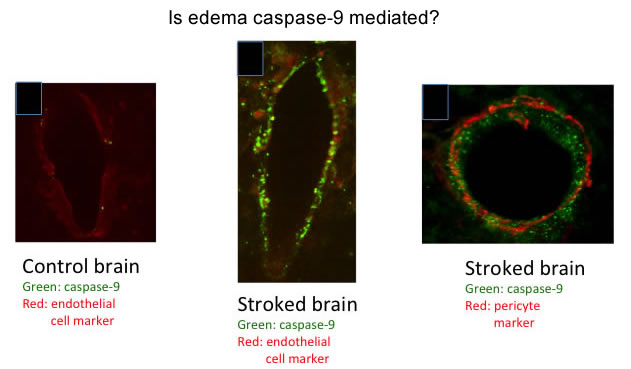 Stroke induces caspase-9 in small blood vessels.
|
The surprising finding of a function for caspase-9 in edema has lead us to develop other models to study edema, to determine if this is a stroke specific function of caspase-9 or if caspase-9 regulates edema in other diseases. Ying Jean is heading a project studying diabetic macular edema using mouse models of retinal edema and of diabetes. Diabetic macular edema (DME) is the major cause of new blindness in people under the age of 50. This work utilizes live imaging techniques, such as fluorescent angiography and ocular coherence tomography (OCT), which enable Ying to follow animals over time, to assess development of edema and efficacy of caspase-9 inhibition. Our specific caspase-9 inhibitor can be delivered topically, as eye drops; current therapeutics for DME require intravitreal delivery, a more invasive approach. Gloria Hong and Sasha White are assisting Ying with his studies. Ying is also studying models of degeneration related to Alzheimer's disease (AD), with projects in collaboration with Ulrich Hengst on ATF4 in AD, and with Joe Lee, Rong Fang, and Richard Mayeux on CUGBP2, an RNA binding protein identified by genome wide association studies (GWAS) in late-onset AD.
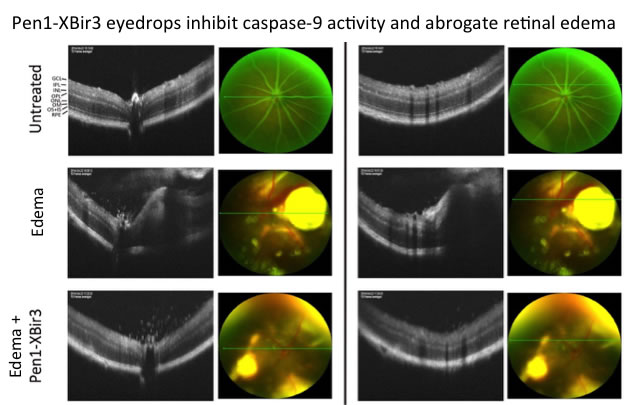 Representative Micron IV Live Images (OCT (black and white) and phase (color)), 2 different levels of OCT are shown for each mouse.
|
Maria Elena Pero is working on determining how low concentrations of beta-amyloid modulate spine dynamics. This work stems from the finding that 300pM Abeta induces an increase in spine density; the increase requires caspase-3 activity and is modulated by the inhibitor of apoptosis family proteins, XIAP and cIAP1. This work suggests that both low concentrations of Abeta and localized, controlled caspase-3 activity are required for optimal functioning of the synapse. These studies utilize in vitro and in vivo approaches. Maya Ramachandran is assisting Maria Elena.

