Columbia University
Irving Medical Center
Neurological Institute
710 West 168th Street, 3rd floor
(212) 305-1818
Featured Research
In the Lab:
Caghan Kizil, MSc, PhD

Caghan Kizil, MSc, PhD
Tissue regeneration is a fascinating phenomenon but it manifests differently in animals. We humans are rather poor regenerators but other vertebrates, such as zebrafish, are stunningly regenerative in various tissues, including the central nervous system. The regenerative capacity of zebrafish brain is largely dependent on the activity of tissue-resident stem cells, which are developmentally and physiologically reminiscent of the stem cells in human brain. However, formation of new neurons—regenerative neurogenesis—is hampered in human disease states while zebrafish can circumvent this.
In our laboratory, we have generated experimental models in zebrafish to mimic certain pathological aspects of Alzheimer’s disease and showed that zebrafish can respond by producing more neurons upon pathology. We demonstrated that this ability relies on a complex set of regulatory cellular mechanisms to sustain the regenerative activity of neural stem/progenitor cells in the zebrafish brain. Activity of the immune system, heterogeneity, and plasticity of the neural stem cell populations, neuron-glia crosstalk, aging, and metabolic state of neurons are among the important determinants of neural regeneration response.
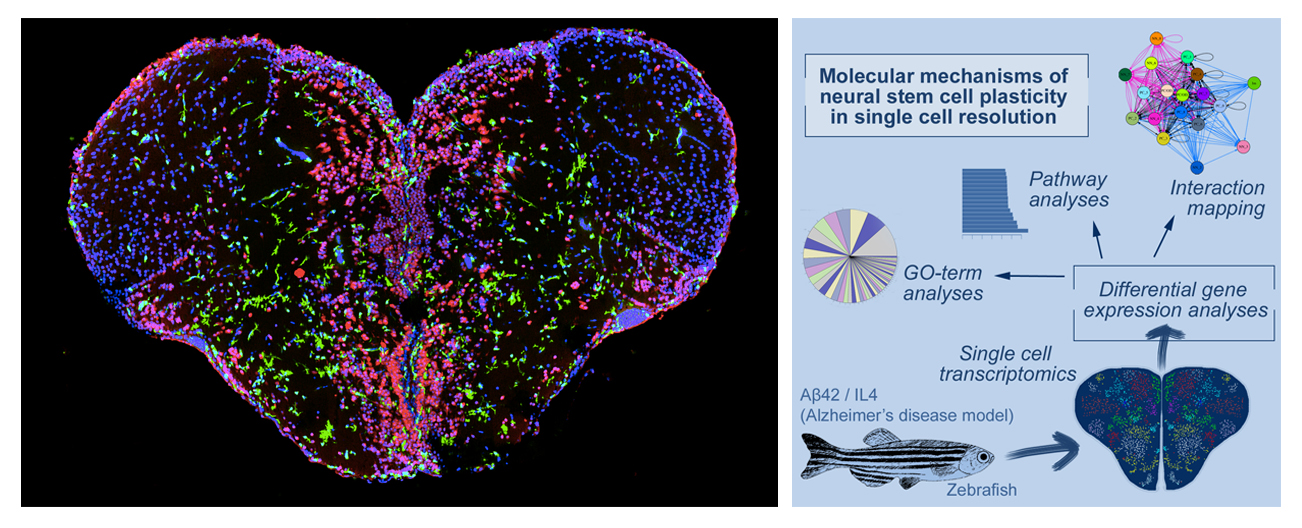
Figure 1. Left: Cross section of adult zebrafish brain. Red: neurons, Green: immune cells, blue: nuclei. Right: single cell sequencing strategy in adult zebrafish brain Alzheimer’s disease model. By using transcriptomics and in silico interaction mapping, we identified new functional subtypes of neural stem cells and progenitors.
We generated the first single cell transcriptomics resource for the adult zebrafish brain in Alzheimer’s-like conditions and identified the pathology-induced functional subsets of neural stem cells in zebrafish brain. Further, we demonstrated that adult zebrafish model of amyloidosis could be used as an in vivo drug screening tool and initiated a comparative investigative pipeline for testing the regenerative programs in zebrafish for their effects in mouse models of AD. We developed an in vitro 3D human neurogenesis and AD pathology model, where we demonstrated the efficiency of zebrafish programs to enhance neurogenesis and response to traumatic injury in vitro.
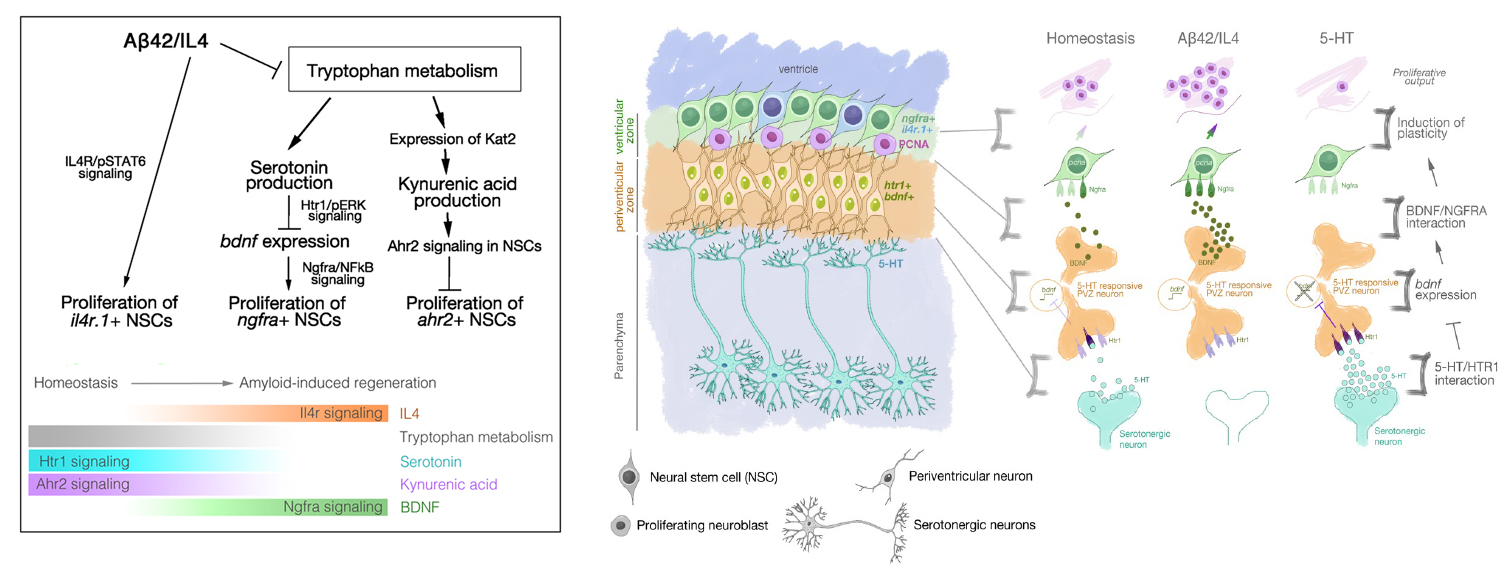
Figure 2. Left: The regenerative neurogenesis response and the plasticity of neural stem cells in adult zebrafish brain are regulated by various molecular signalling pathways including IL4, BDNF, KYNA and their receptors present in functional subsets of glia. Right: Example of neuron-glia crosstalk through Serotonin-BDNF/NGFR axis that regulates amyloid-induced neurogenesis.
Collectively, our studies suggest that the key success of neuronal regeneration is to coordinate a set of molecular programs in the right cell type at the right time, through a set of favorable cellular interactions. Thus, our lab’s goal has been to find out the detailed molecular basis of how neural stem cells become regenerative in zebrafish, how new neurons survive and integrate into the circuitry, and how proliferation-differentiation-integration cascade is successfully executed. Yet, there is a lot to learn from zebrafish. We hypothesized that if we could understand the regenerative programs in a spatiotemporal manner, we could harness this knowledge for designing regenerative therapies in humans in clinical settings. Our innovative question is whether we can use the regenerative molecular programs for designing a regenerative route as an intervention to neurodegenerative diseases in humans.
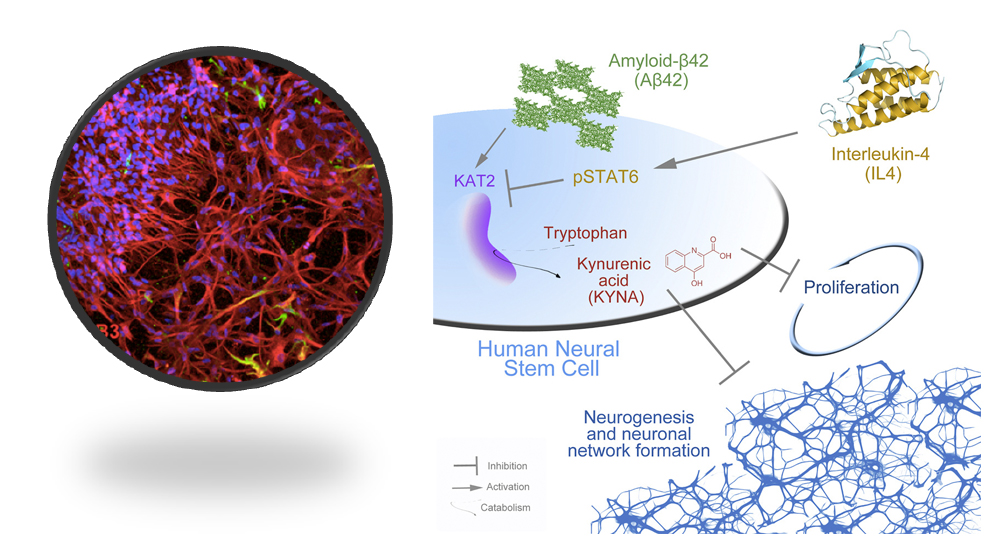
Figure 3. Left: 3D human neural stem cell-derived cortical neuronal cultures in instructive hydrogels. Red: neurons, Green: astrocytes, Blue: nuclei. Right: Similar to zebrafish brain, Interleukin-4 rescues the amyloid-dependent reduction of human neural stem cell plasticity through suppressing Kynurenic acid production in 3D human neural stem cell plasticity model, demonstrating that zebrafish brain can be used as a reductionist experimental system for predicting molecular changes in Alzheimer’s disease.
I joined the Taub Institute and the Department of Neurology as a visiting professor after being awarded the VP&S Schaefer Research Scholars Award in 2021. With this award, we are investigating the role of Alzheimer's disease variants in regenerative neurogenesis. The rich, multidisciplinary scientific and clinical environment at Columbia University Irving Medical Center has helped me establish many fruitful collaborations. I aim to establish a rooted, cross-disciplinary research agenda that will include integrating zebrafish to functional validation of clinical findings in Alzheimer’s disease, to improve understanding of the roadblocks to neural regeneration in humans.
Caghan Kizil, PhD
Visiting Professor of Neurological Sciences (in Neurology and the Taub Institute)
ck2893@cumc.columbia.edu
Tenured W2 Appointment, DZNE, Dresden, Germany
caghan.kizil@dzne.de

